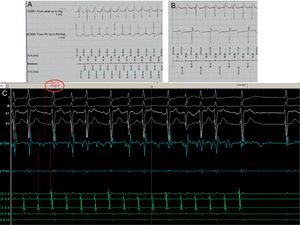
We report the case of a 41-year-old woman with chronic rheumatoid arthritis, who was treated in 2007 with ablation of 2 focal atrial tachycardias in the ostium of the coronary sinus and peri-Hisian region. A further study was performed in 2012 due to palpitations and nausea, which revealed severe sinus node dysfunction and paroxysmal atrioventricular block (AVB), without tachyarrhythmia induction. A dual-chamber pacemaker was implanted. In 2015, she developed an exacerbation of rheumatoid arthritis, followed by rapid palpitations and presyncope. The pacemaker showed multiple episodes of atrial tachycardia, which alternated 1:1 conduction with AVB, and required ventricular pacing (Figure 1A and Figure 1B). Furthermore, since 2016, a high degree of variability has been observed in the atrial electrode sensing and threshold, ranging from complete absence of atrial activity with complete failure to capture, to other moments with normal sensing and threshold. Electroanatomical mapping revealed the absence of electrical activity in practically the whole left atrium (LA), and in the septal area of the right atrium (RA). We were able to induce clinical tachycardia and ablate it in the medial-septal area of the RA (Figure 1C). The results of laboratory tests, including reagents and autoantibodies, were negative. Cardiac magnetic resonance revealed extensive fibrosis in the LA and focal fibrosis in the RA (where radiofrequency had previously been applied); there was no delayed enhancement in the rest of the heart. These findings were in line with electroanatomical mapping (Figure 2). The patient remained asymptomatic during the follow-up period.
A: Atrial tachycardia at a cycle of 260-270ms with 1:1 conduction to the ventricle at 222-230 bpm. B: Atrial tachycardia with similar morphology and cycle of 300-330ms, with atrioventricular block and pacemaker pacing. C: Endocavity registers in the EPS at 50mm/s; with 4 signals in the ECG (LI, LIII, V1 and V2), 2 bipolar signals in the mapping/ablation catheter register, and 5 signals in the CS (1-2 distal; 9-10 proximal). Atrial tachycardia was observed with irregular conduction to the ventricle, eccentric activation of the CS, and earlier activation in the mapping/ablation catheter compared with the initial P wave. This is explained by the LA fibrosis (which caused slow conduction) and the fact that the previous ablations in the ostium had isolated the proximal portion of the CS. As a result, during tachycardia, the LA depolarizes through the superior portion (Bachmann's bundle) and reaches the CS from the lateral portion, giving rise to this eccentric activation. The application of radiofrequency at this point inhibits the tachycardia by restoring sinus rhythm. EGM1, bipolar atrial electrogram between the tip and the ring of the atrial electrode; EGM2, bipolar ventricular electrogram between the tip of the RV (RV tip) and the ring of the RV (RV ring). Ab, atrial signal detected during the atrial blanking period; AP, atrial paced event; AR, atrial refractory period; AS, atrial sensed event; CS, coronary sinus; ECG, electrocardiogram; EGM, electrogram; EPS, electrophysiology study; LA, left atrium; RV, right ventricle; VP, ventricular paced event; VS, ventricular sensed event.
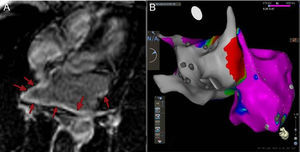
Atrial images showing extensive atrial fibrosis. A: Image of 4 chambers of the LA with cardiac magnetic resonance imaging, with diffuse delayed enhancement (arrows). B: Posterior view of the bipolar electroanatomical mapping of both atria. The color scale indicates the voltage of the electrograms recorded with the endocardial catheter during the study. Voltage < 0.1mV (grey) consistent with fibrosis, and voltage > 0.5mV (pink) suggestive of normal tissue. The image shows a highly pathological LA while the RA is practically normal. LA, left atrium.
Rheumatoid arthritis has been associated with cardiac damage due to inflammation at all levels,1 mainly in the form of heart disease, but also due to conduction tissue damage with AVB2 and severe arrhythmia.3 Magnetic resonance studies have shown frequent myocardial abnormalities, even in asymptomatic patients, associated with increased arthritis activity. As a result, the role of inflammation in the heart is increased.4
What is notable about this case is the age at which the patient showed severe extensive atrial fibrosis, together with multiple atrial arrhythmias (bradycardia-tachycardia syndrome) and AVB. The patient also experienced paroxysmal episodes, which were reproducible and simultaneous with atrial standstill and failure to capture, and the electrode was functioning normally. We evaluated the electrode dysfunction and considered placement of a new one. However, as this was a fluctuating abnormality which always coincided with the absence of atrial activity, and normal impedances, which were reproducible during the electrophysiology study, we believed that it would depend more on the atrial myocardium or the electrode-myocardium interface, rather than on the electrode itself. A general explanation for the fluctuation, fibrosis, and arrhythmias could be an acute exacerbation of the rheumatoid arthritis (as the palpitations began with an exacerbation some months previously), but at the time of the study the patient had no clinical symptoms and the reagents were normal. The inflammation could therefore have acted as a triggering factor, and the severe extensive secondary fibrosis could act as the substrate to produce these events, in the form of “subacute/chronic atrial myocarditis”.
To summarize, this is a young patient with rheumatoid arthritis who showed cardiac involvement in the form of arrhythmias, with paroxysmal supra-Hisian AVB, and bradycardia-tachycardia syndrome, attributable to extensive atrial fibrosis. This affected pacemaker function, with erratic behavior, and atrial standstill coinciding with a complete failure to capture. This was probably a translation of an “atrial myocarditis”-type condition, which made it completely unexcitable, and which has not previously been described.