Degenerative aortic stenosis (AS) is the leading cause of valvular heart disease in our setting. Prevalence is increasing due to longer life expectancy and gradual population aging.1 Transcatheter aortic valve implantation (TAVI) is a safe and effective treatment for severe degenerative AS in patients in whom surgery is high risk or contraindicated. The main studies assessing the effectiveness of TAVI have not specifically analyzed the outcome of TAVI in very elderly patients.2–4 The aim of this study was to describe the safety and efficacy of TAVI in nonagenarian patients with major functional limitations secondary to severe degenerative AS.
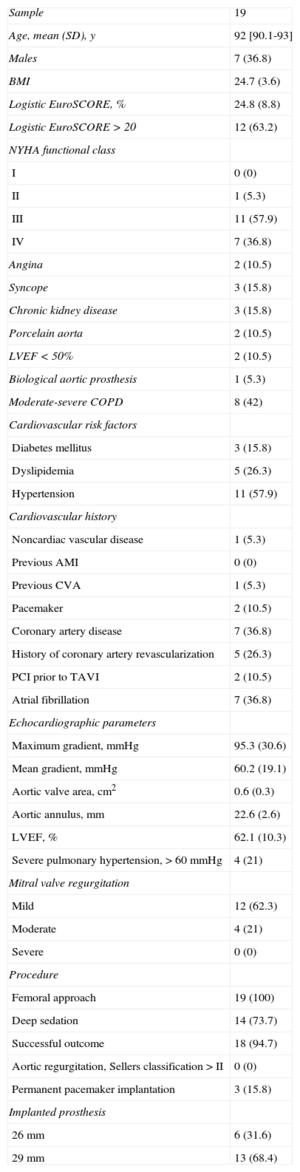
We conducted a multicenter, prospective, observational study from December 2008 until April 2012, enrolling 19 consecutive patients with severe degenerative AS aged 90 years and older from 3 hospitals in Spain: Hospital Virgen de la Victoria in Malaga (n=8), Hospital Universitario Central de Asturias in Oviedo (n=6), and the Complexo Hospitalario Universitario in Santiago de Compostela (n=5). All patients were implanted with a self-expanding CoreValve prosthesis (Medtronic; Minneapolis, Minnesota, United States). We included patients aged 90 years and older with severe, symptomatic AS, who met anatomic implantation requirements as described in the literature.3,5 Surgical risk was assessed using the logistic EuroSCORE. A multidisciplinary team1 assessed each case to determine the indication for TAVI. We used the SPSS software package for basic descriptive statistics and to perform a Kaplan-Meier survival analysis. The baseline characteristics of the study population are shown in the Table.
Baseline Characteristics of the Study Population
| Sample | 19 |
| Age, mean (SD), y | 92 [90.1-93] |
| Males | 7 (36.8) |
| BMI | 24.7 (3.6) |
| Logistic EuroSCORE, % | 24.8 (8.8) |
| Logistic EuroSCORE > 20 | 12 (63.2) |
| NYHA functional class | |
| I | 0 (0) |
| II | 1 (5.3) |
| III | 11 (57.9) |
| IV | 7 (36.8) |
| Angina | 2 (10.5) |
| Syncope | 3 (15.8) |
| Chronic kidney disease | 3 (15.8) |
| Porcelain aorta | 2 (10.5) |
| LVEF < 50% | 2 (10.5) |
| Biological aortic prosthesis | 1 (5.3) |
| Moderate-severe COPD | 8 (42) |
| Cardiovascular risk factors | |
| Diabetes mellitus | 3 (15.8) |
| Dyslipidemia | 5 (26.3) |
| Hypertension | 11 (57.9) |
| Cardiovascular history | |
| Noncardiac vascular disease | 1 (5.3) |
| Previous AMI | 0 (0) |
| Previous CVA | 1 (5.3) |
| Pacemaker | 2 (10.5) |
| Coronary artery disease | 7 (36.8) |
| History of coronary artery revascularization | 5 (26.3) |
| PCI prior to TAVI | 2 (10.5) |
| Atrial fibrillation | 7 (36.8) |
| Echocardiographic parameters | |
| Maximum gradient, mmHg | 95.3 (30.6) |
| Mean gradient, mmHg | 60.2 (19.1) |
| Aortic valve area, cm2 | 0.6 (0.3) |
| Aortic annulus, mm | 22.6 (2.6) |
| LVEF, % | 62.1 (10.3) |
| Severe pulmonary hypertension, > 60 mmHg | 4 (21) |
| Mitral valve regurgitation | |
| Mild | 12 (62.3) |
| Moderate | 4 (21) |
| Severe | 0 (0) |
| Procedure | |
| Femoral approach | 19 (100) |
| Deep sedation | 14 (73.7) |
| Successful outcome | 18 (94.7) |
| Aortic regurgitation, Sellers classification > II | 0 (0) |
| Permanent pacemaker implantation | 3 (15.8) |
| Implanted prosthesis | |
| 26 mm | 6 (31.6) |
| 29 mm | 13 (68.4) |
AMI, acute myocardial infarction; BMI, body mass index; CVA, cerebrovascular accident; EuroSCORE, European System for Cardiac Operative Risk Evaluation; ICP, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; TAVI, transcatheter aortic valve implantation.
Data are expressed as median [interquartile range], mean (standard deviation) or No. (%).
There were 2 major vascular complications, which were common femoral occlusion after percutaneous closure, solved by implanting a coated stent, and acute right iliac occlusion on valve removal, which required emergency vascular surgery and resulted in the patient's death. There were no other complications. Mean hospital stay was 10.8 days (standard deviation, 4 days).
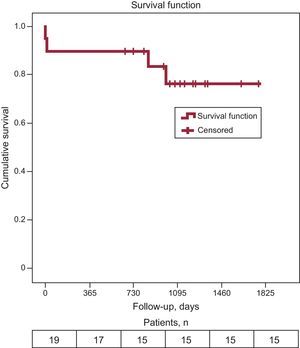
Follow up was performed onsite or by telephone (February 2014). During the follow-up period (median, 1080 days [interquartile range, 823-1329 days]), there were no cerebrovascular accidents and 4 deaths, 2 of which were of cardiovascular origin and occurred in hospital (1 as described above for acute iliac occlusion and 1 due to refractory heart failure and multiple organ failure). The other 2 deaths were not cardiovascular-related and occurred at 846 days and 999 days post-implantation. Total survival (Figure) was 76.2%, with an estimated median survival of 1472 days (95% confidence interval, 1203.8-1740.1 days).
Our case series shows that TAVI treatment in heart team-selected nonagenarian patients with severe, limiting, degenerative AS is safe and effective, despite their high-risk classification determined using the EuroSCORE.5 These patients are usually treated conservatively, regardless of the poor prognosis associated with medical treatment. In this respect, the results observed in our study are of key clinical importance because, in very elderly patients, age alone may be the main criterion to contraindicate surgery in the absence of other significant comorbidities (the EuroSCORE places considerable weight on advanced age). In our setting, in which life expectancy is increasing, it is common to see elderly patients with good quality of life, principally limited by a degenerative disease such as severe AS.6 We observed a high success rate for TAVI, matching data published in other series in which age was not a selection criterion. Complications post-implantation were also similar. Mortality mainly occurs during the hospital stay and is clearly dependent on the success rate.3,4
In view of the population characteristics in our setting, it is likely that we will be caring for an increasing number of very elderly patients and TAVI may be an attractive alternative for those who do not have major comorbidities and whose functional limitations are mainly due to severe degenerative AS.