The PEACE study (Performance of a sirolimus-eluting balloon strategy in acute and chronic coronary syndromes) investigated for the first time whether a sirolimus-coated balloon (SCB) (Magic Touch, Concept Medical, India) is associated with different outcomes depending on whether it is used in acute coronary syndromes (ACS) or chronic coronary syndromes (CCS).
MethodsThis was a post-hoc analysis from the all-comers EASTBOURNE Registry (NCT03085823). Out of 2083 patients enrolled, an SCB was used to treat 968 (46.5%) ACS and 1115 (53.5%) CCS patients. The primary endpoint was target lesion revascularization at 12 months, while secondary endpoints were angiographic success and major adverse cardiovascular events.
ResultsBaseline demographics, mean reference vessel diameter and mean lesion length were comparable between ACS and CCS. Predilatation was more commonly performed in ACS (P=.007). SCB was inflated at a standard pressure in both groups with a slight trend toward longer inflation time in ACS. Angiographic success was high in both groups (ACS 97.4% vs CCS 97.7%, P=.820) with limited bailout stenting. Similarly, at 12 months the cumulative incidence of target lesion revascularization (ACS 6.6% vs CCS 5.2%, P=.258) was comparable between ACS and CCS. Conversely, a higher rate of major adverse cardiovascular events in acute presenters was mainly driven by myocardial infarction recurrencies (ACS 10.4% vs CCS 8.3%, P=.009). In-stent restenosis showed a higher proportion of target lesion revascularization and major adverse cardiovascular events than de novo lesions, independently of the type of presentation at the index procedure.
ConclusionsThis SCB shows good performance in terms of acute and 1-year outcomes independently of the clinical presentation.
Keywords
The use of drug-coated balloons (DCB) to treat coronary artery disease has been expanding progressively in recent years. A nonnegligible rate of target vessel failure in the modern era of drug-eluting stents (DES) has created the need for different treatment strategies during percutaneous coronary intervention (PCI).1,2
When compared with DES, DCBs do not leave any metallic scaffolding in the vessel and provide fast and homogeneous transfer of antiproliferative drugs into the vessel wall upon balloon inflation. This treatment preserves coronary vasomotion, may promote positive vessel remodelling, and potentially translates into reduced late lumen loss at follow-up.3
The most widely accepted fields of application for DCB are in-stent restenosis (ISR), small vessels and combined strategies with DES during complex PCI (such as bifurcations).4–9 The available data mainly refer to paclitaxel-based platforms; it is only more recently that the introduction of new technologies for coating balloons including -limus family drugs has led to some preclinical and clinical studies using sirolimus-based platforms, thus opening the way to their use in clinical practice.
Overall, DCB are not routinely used to treat native large-vessel disease, since evidence is limited, and new-generation DES remain the gold standard following the international guidelines. Similarly, chronic total occlusions and acute coronary syndromes (ACS) still represent a gray zone for DCB use and further information from randomized clinical trials is needed.
As for the latter group, limited data are available. It has been questioned that drug delivery may be impaired in thrombus-containing lesions10; additionally, the need for mandatory lesion preparation before DCB, as well as for prolonged balloon inflation, may potentially discourage their use in acute settings where the risk of no-reflow is high. However, thrombus itself may also prevent correct vessel sizing, which paves the way to stent malapposition; in addition, the inflammatory milieu of ACS may favor delayed tissue coverage. Accordingly, both these conditions may benefit from the lack of stent struts, especially in multivessel coronary disease where multiple revascularizations are anticipated.
The all-comers EASTBOURNE Registry aims to evaluate the performance of a novel sirolimus-coated balloon in a large series of patients (NCT03085823).11 The PEACE study (Performance of a sirolimus-eluting balloon strategy in acute and chronic coronary syndromes) is a post-hoc analysis that compares DCB performance in chronic and acute settings.
METHODSDevice descriptionThe Magic Touch balloon catheter (Concept Medical, India) is a rapid exchange, semicompliant, sirolimus-coated balloon based on the Nanolute technology. Sirolimus has poor lipophilicity and can be easily lost in the bloodstream. The Nanolute technology is intended to overcome these limitations. More specifically, sirolimus submicron particles are encapsulated in a bilayer phospholipid drug carrier (the nanocarrier). An inert gas-assisted spray process provides circumferential balloon coating with sirolimus nanocarriers. The drug concentration reaches 1.27 μg/mm2. Upon balloon inflation at the target site, nanocarriers are released and, due to pH variation, releases sirolimus, which then penetrates the layers of the vessel wall.12
Available balloon sizes go up to 40 mm in length and 4 mm in diameter and are all compatible with standard 0.014” wires and 6 Fr guiding catheters.
EASTBOURNE study designThe EASTBOURNE registry was a prospective, multicenter, investigator-driven, clinical registry enrolling a real-world, all-comer population treated with the Magic Touch sirolimus-eluting balloon. The aim of this registry was to observe and evaluate the performance of the sirolimus-coated balloon Magic Touch for the treatment of any type of coronary lesion.13 The study was conducted in accordance with the Declaration of Helsinki and each enrolled patient signed an informed consent form. The demographics of the study population were collected including the sex variable which was self-reported.
Thirty-eight centers in Europe and Asia with adequate experience in DCB use were involved (each investigator had to certify the use of at least 30 DCB per year in the last 5 years). All patients aged more than 18 years-old and with an indication for PCI were eligible for inclusion. The decision to use DCB or any other treatment strategy was left to the operator's choice. Lesion evaluation was based on visual assessment by the single operator. Apart from known hypersensitivity to sirolimus or other procedure related drugs, the only exclusion criteria were the presence of severe calcifications and tortuosity at the target vessel and severe thrombotic burden not treatable with manual aspiration.
As recommended by the third report of the International DCB Consensus Group, lesion preparation was strongly encouraged with any device deemed appropriate and unsuccessful predilatation was an exclusion criterion for DCB treatment.3
Prolonged DCB inflation was encouraged for at least 30seconds and preferably for 60seconds. The decision to implant a stent after DCB was left to the discretion of the operator but was recommended only if there was residual at least type C dissection and reduced thrombolysis in myocardial Infarction flow.
The study design included a clinical evaluation (telephone call or visit) at 1, 6, 24 and 36 months and an office visit at 12 months after the procedure.
The primary endpoint was clinically indicated target lesion revascularization (TLR) at 12 months. Secondary endpoints were angiographic success (residual stenosis less than 50% and thrombolysis in myocardial Infarction 3 coronary flow), procedural success (angiographic success and absence of adverse cardiovascular events during hospitalization) and major adverse cardiac events (MACE), a composite of cardiac death, acute spontaneous myocardial infarction (MI) and need for TLR at 6, 12, 24, and 36 months of implantation. A centralized event committee composed of cardiologists not participating at the study evaluated all the events in the electronic case report form after having obtained the relative documentation.
PEACE substudyThe PEACE substudy was a post-hoc cohort analysis to compare the performance of the Magic Touch device in ACS vs chronic coronary syndromes (CCS) based on the clinical presentation at the index procedure. ACS included patients presenting with unstable angina, non-ST-elevation MI (NSTEMI) and ST-elevation MI (STEMI), while CCS included patients with stable angina and silent ischemia. Both de novo lesions and ISR were included in the analysis. After comparing baseline and procedural characteristics between the 2 groups, we focused on in-hospital (angiographic success) and 12-month outcomes (need for TLR and occurrence of MACE).
Statistical analysisCategorical variables are reported as count and percentage, and continuous variables as the mean±standard deviation (SD) or median [interquartile range (IQR)]. The Student t test was used to assess differences between parametric continuous variables, the Mann-Whitney U test for nonparametric variables, and the chi-square test or Fisher exact test for categorical variables. The overall cumulative risk of TLR was estimated using the Kaplan-Meier method, and differences among groups with the log-rank test. Comparison of outcome was evaluated between CCS and ACS and thereafter stratified according to the type of target lesion (de novo vs ISR). The effect of clinical indication for PCI on study endpoints was estimated by a Cox proportional hazards model and expressed as a hazard ratio (HR), 95% confidence interval (95%CI) and P value. Adjusted models were used based on clinical and procedural characteristics with statistical significance, which included age, sex, dyslipidemia, previous MI, previous PCI, left ventricular ejection fraction, creatinine, hemoglobin, predilatation, SCB pressure of inflation, and SCB time of inflation. A 2 -sided P<.05 was considered statistically significant; all analyses were performed using the R software (R Core Team 2022, R Foundation for Statistical Computing, Austria).
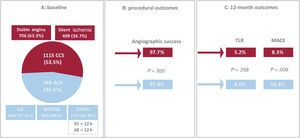
RESULTSOverall, 2123 patients treated with Magic Touch DCB were enrolled in the EASTBOURNE registry between September 2016 and November 2020. The clinical presentation at the index procedure was not reported in 40 patients, so that 2083 patients were finally included in the PEACE substudy. This population included 1115 (53.5%) CCS patients and 968 (46.5%) ACS patients treated with the study device. There were 364 patients with unstable angina (37.6%), 445 with NSTEMI (46%) and 159 with STEMI (16.4%, including early and late presenters belonged to the latter group) (figure 1A). A total of 79% of the lesions included in the ACS group were deemed culprit lesions by the investigator. The 12-month follow-up information was completed in 1927 patients (92.5%).
Central illustration. PEACE substudy population with procedural and 12-month outcomes. A. overall population at baseline according to the clinical presentation at the index procedure. B. immediate procedural outcomes in terms of angiographic success (residual stenosis less than 50% and thrombolysis in myocardial infarction 3 coronary flow) compared between ACS and CCS patients. C. 12-month outcomes in terms of TLR and MACE compared between ACS and CCS patients. ACS, acute coronary syndrome; CCS, chronic coronary syndrome; MACE, major adverse cardiovascular event; NSTEMI, non–ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction; TLR, target lesion revascularization; UA, unstable angina.
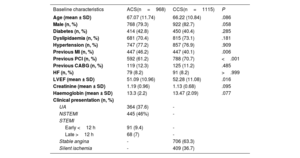
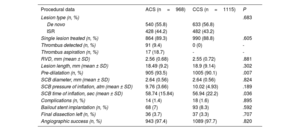
Baseline demographics were comparable between ACS and CCS patients, except for the prevalence of previous MI, which was higher for acute presentations (ACS 46.2% vs CCS 40.1%, P=.006) (table 1). De novo lesions represented more than half of the total study population (56.3%) and were equally distributed between the 2 groups (P=.683) (figure 2A). Procedural characteristics are reported in table 2. A DCB strategy was applied to a single lesion in most patients (89.3% vs 88.8% in ACS vs CCS patients respectively, P=.695), but up to 4 lesions were eventually treated with DCB in a few cases. As assessed by fluoroscopy, mildly and moderately calcified lesions were more common among ACS and CCS patients respectively, while severely calcified lesions were equally represented (mild 61.2% vs 9.4%; moderate 31.8% vs 84.4%; severe 7.1% vs 6.2%; P≤.001). Mean reference vessel diameter as well as mean lesion length were comparable between the 2 groups (diameter 2.56±0.68 vs 2.55±0.72 respectively, P=.881; length 18.49±9.20 vs 18.9±9.14 respectively, P=.302). Notably, predilatation was performed in a high proportion but not in all patients, despite strong recommendation per protocol, and was more commonly used in ACS than in CCS patients (93.5% vs 90.1%, P=.007). DCB were inflated at a standard pressure in both groups (mean atmospheres 9.76±3.66 vs 10.02±4.93, P=.189) with a moderately longer inflation time in acute coronary syndromes (mean duration expressed in seconds 58.74±15.84 vs 56.94±22.2, P=.036).
Baseline characteristics compared between acute coronary syndrome and chronic coronary syndrome patients in the overall population
| Baseline characteristics | ACS(n=968) | CCS(n=1115) | P |
|---|---|---|---|
| Age (mean ± SD) | 67.07 (11.74) | 66.22 (10.84) | .086 |
| Male (n, %) | 768 (79.3) | 922 (82.7) | .058 |
| Diabetes (n, %) | 414 (42.8) | 450 (40.4) | .285 |
| Dyslipidaemia (n, %) | 681 (70.4) | 815 (73.1) | .181 |
| Hypertension (n, %) | 747 (77.2) | 857 (76.9) | .909 |
| Previous MI (n, %) | 447 (46.2) | 447 (40.1) | .006 |
| Previous PCI (n, %) | 592 (61.2) | 788 (70.7) | <.001 |
| Previous CABG (n, %) | 119 (12.3) | 125 (11.2) | .485 |
| HF (n, %) | 79 (8.2) | 91 (8.2) | >.999 |
| LVEF (mean ± SD) | 51.09 (10.96) | 52.28 (11.08) | .016 |
| Creatinine (mean ± SD) | 1.19 (0.96) | 1.13 (0.68) | .095 |
| Haemoglobin (mean ± SD) | 13.3 (2.2) | 13.47 (2.09) | .077 |
| Clinical presentation (n, %) | |||
| UA | 364 (37.6) | - | |
| NSTEMI | 445 (46%) | - | |
| STEMI | |||
| Early <12 h | 91 (9.4) | - | |
| Late >12 h | 68 (7) | - | |
| Stable angina | - | 706 (63.3) | |
| Silent ischemia | - | 409 (36.7) |
ACS, acute coronary syndrome; CABG, coronary artery by-pass graft; CCS, chronic coronary syndrome; HF, heart failure; LVEF, left ventricle ejection fraction; MI, myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction; UA, unstable angina.
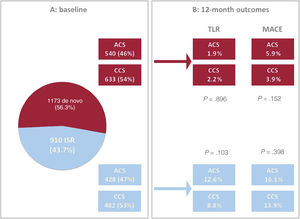
Twelve-month outcomes of the PEACE cohort of patients (ACS and CCS) according to the type of lesion treated (de novo and ISR). A: overall population at baseline according to type of lesion treated. B: 12-month outcomes in terms of TLR and MACE according to the type of lesion treated and compared between ACS and CCS patients. ACS, acute coronary syndrome; CCS, chronic coronary syndrome; ISR, in-stent restenosis; MACE, major adverse cardiovascular event TLR, target lesion revascularization.
Procedural data compared between acute coronary syndrome and chronic coronary syndrome patients in the overall population
| Procedural data | ACS (n=968) | CCS (n=1115) | P |
|---|---|---|---|
| Lesion type (n, %) | .683 | ||
| De novo | 540 (55.8) | 633 (56.8) | |
| ISR | 428 (44.2) | 482 (43.2) | |
| Single lesion treated (n, %) | 864 (89.3) | 990 (88.8) | .605 |
| Thrombus detected (n, %) | 91 (9.4) | 0 (0) | - |
| Thrombus aspiration (n, %) | 17 (18.7) | - | - |
| RVD, mm (mean ± SD) | 2.56 (0.68) | 2.55 (0.72) | .881 |
| Lesion length, mm (mean ± SD) | 18.49 (9.2) | 18.9 (9.14) | .302 |
| Pre-dilatation (n, %) | 905 (93.5) | 1005 (90.1) | .007 |
| SCB diameter, mm (mean ± SD) | 2.64 (0.56) | 2.64 (0.56) | .824 |
| SCB pressure of inflation, atm (mean ± SD) | 9.76 (3.66) | 10.02 (4.93) | .189 |
| SCB time of inflation, sec (mean ± SD) | 58.74 (15.84) | 56.94 (22.2) | .036 |
| Complications (n, %) | 14 (1.4) | 18 (1.6) | .895 |
| Bailout stent implantation (n, %) | 68 (7) | 93 (8.3) | .592 |
| Final dissection left (n, %) | 36 (3.7) | 37 (3.3) | .707 |
| Angiographic success (n, %) | 943 (97.4) | 1089 (97.7) | .820 |
ISR, in-stent restenosis; RVD, reference vessel diameter; SCB, sirolimus coated balloon.
Lesion characteristics refer to the main lesion in case of multiple lesions treated.
Angiographic success was high in both groups (ACS 97.4% vs CCS 97.7%, P=.820) (figure 1B) and stent implantation was limited to a low proportion of patients (ACS, n=68, 7% vs CCS, n=93, 8.3%; P=.592) for acute vessel recoil or flow-limiting dissection. Overall, a final dissection as detected on angiography was left in 73 patients (36 ACS, 3.7% vs 37 CCS, 3.3%; P=.707).
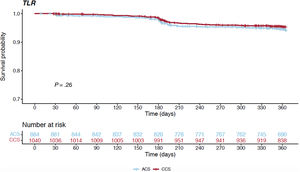
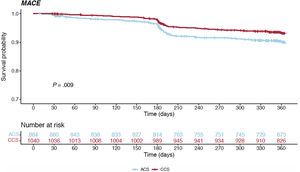
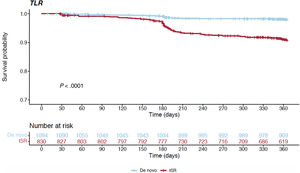
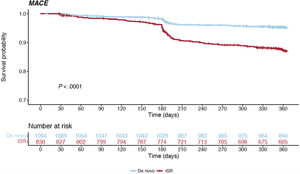
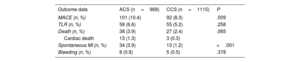
Table 3 and figure 1C report the overall 1-year unadjusted outcomes. At 12 months, the cumulative incidence of TLR was comparable irrespective of whether the index procedure had been performed in an acute or a chronic setting (6.6% vs 5.2% respectively, HR, 0.792; 95%CI, 0.528-1.186; P=.258) (figure 3); conversely, the cumulative incidence of MACE was higher for ACS presenters (10.4% vs 8.3%, HR, 0.656; 95%CI, 0.477-0.902; P=.009) and its occurrence seemed to diverge from the 6-month follow-up onwards (figure 4). The difference in the composite endpoint was mainly driven by a higher incidence of spontaneous MI (3.9% vs 1.2%, P<.001).
12 months outcomes compared between acute coronary syndrome and chronic coronary syndrome patients in the overall population
| Outcome data | ACS (n=968) | CCS (n=1115) | P |
|---|---|---|---|
| MACE (n, %) | 101 (10.4) | 92 (8.3) | .009 |
| TLR (n, %) | 58 (6.6) | 55 (5.2) | .258 |
| Death (n, %) | 38 (3.9) | 27 (2.4) | .065 |
| Cardiac death | 13 (1.3) | 3 (0.3) | |
| Spontaneous MI (n, %) | 34 (3.9) | 13 (1.2) | <.001 |
| Bleeding (n, %) | 8 (0.9) | 5 (0.5) | .378 |
ACS, acute coronary syndrome; CCS, chronic coronary syndrome; MACE, major adverse cardiovascular events; MI, myocardial infarction; TLR, target lesion revascularization.
After adjustment of study outcomes for baseline and procedural characteristics, the overall study results were confirmed (TLR: HR, 0.76, P=.202; MACE: HR, 0.70, P=.036).
Subgroup analyses of the ISR and de novo lesions were also performed (patients with ACS treated for ISR 428, 47%; patients with CCS treated for ISR 482, 53%). At 12 months of follow-up, ISR treated with DCB showed a higher proportion of TLR and MACE occurrence compared with de novo lesions treated with a similar strategy (figure 5 and figure 6), independently of the type of presentation at the index procedure (for more details see table 4 and figure 2B). Similarly, it is noteworthy that recurrent spontaneous MI (a single component of the composite endpoint) was more common in ACS than CCS patients treated for ISR rather than de novo lesions (12-month cumulative rate of MI for de novo lesions: 1.2% vs 0.8% in ACS vs CCS, P=.748; 12-month cumulative rate of MI for ISR: 7.2% vs 1.8% in ACS vs CCS, P<.001).
12 months outcomes compared between acute coronary syndrome and chronic coronary syndrome patients treated for either de novo coronary lesions or in-stent restenosis lesions
| De novo coronary lesions (n=1173) | |||
|---|---|---|---|
| Outcome data | ACS (n=540) | CCS (n=633) | P value |
| MACE (n, %) | 32 (5.9) | 25 (3.9) | .152 |
| TLR (n, %) | 9 (1.8) | 13 (2.2) | .851 |
| Death (n, %) | 16 (3.0) | 8 (1.3) | .065 |
| Cardiac death | 3 (0.6) | 0 (0) | |
| MI (n, %) | 6 (1.2) | 5 (0.8) | .748 |
| Bleeding (n, %) | 3 (0.6) | 3 (0.5) | >.999 |
| ISR lesions (n=910) | |||
|---|---|---|---|
| Outcome data | ACS (n=428) | CCS (n=482) | P value |
| MACE (n, %) | 69 (16.1) | 67 (13.9) | .398 |
| TLR (n, %) | 49 (12.6) | 42 (9.2) | .141 |
| Death (n, %) | 22 (5.1) | 19 (3.9) | .478 |
| Cardiac death | 10 (2.3) | 3 (0.6) | |
| MI (n, %) | 28 (7.2) | 8 (1.8) | <.001 |
| Bleeding (n, %) | 5 (1.3) | 2 (0.4) | .330 |
ACS, acute coronary syndrome; CCS, chronic coronary syndrome; ISR, in-stent restenosis; MACE, major adverse cardiovascular events; MI, myocardial infarction; TLR, target lesion revascularization.
Percutaneous reperfusion strategies have dramatically improved outcomes in patients presenting with acute MI, and stent implantation has progressively overcome plain old balloon angioplasty by reducing the need for TLR at follow-up.14 Nevertheless, even new generation DES do not significantly reduce the incidence of hard endpoints such as mortality or recurrent MI during long-term follow-up, which has been explained by the possible occurrence of stent-related complications (late and very-late thrombosis).15
As an alternative, a DCB strategy combines the advantages of an antiproliferative drug with the absence of a metallic permanent scaffolding of the vessel.
The main findings of the present study can be summarized as follows: a) this is the first study investigating the performance of a sirolimus DCB in acute coronary syndromes, given that only paclitaxel-based platforms have previously been validated in this setting; b) the Magic Touch sirolimus DCB shows similar performance in terms of acute and 1-year outcomes whether used for PCI during ACS or CCS; c) similar predilatation rates and DCB inflation times between ACS and CCS suggest that longer procedural times, as expected when using DCB, do not prevent operators from applying this strategy even in the acute setting.
As already mentioned, evidence on the application of DCB to ACS patients are limited to some prospective registries and a few randomized trials, all based on the use of paclitaxel-eluting balloons.16–19The REVELATION (Revascularization with paclitaxel-coated balloon angioplasty vs drug-eluting stenting in acute MI) trial enrolled 120 STEMI patients eligible for primary PCI who were randomized 1:1 to either treatment with DCB (Pantera Lux, Biotronik, Germany) or DES (Orsiro, Biotronik; or Xience, Abbott, Abbott Park, United States). The angiographic follow-up at 9 months showed that the primary endpoint fractional flow reserve did not differ between the 2 groups (P=.27); similarly, there was no difference in the clinical secondary composite endpoint MACE (P=1.0), which also included TLR (3% for DEB vs 2% for DES).19 A similar MACE rate between the 2 groups was confirmed at 2 years of follow-up (hazard ratio, 2.86; 95%CI, 0.30-27.53; P=.34). Only 1 additional event was reported between the 9-month and 2-year follow-up. The specific patient was initially allocated to DCB angioplasty but required bailout stenting during the index procedure and TLR occurred due to ISR leading to an acute coronary syndrome at 561 days after implantation.20
The PEP-CAD NSTEMI trial enrolled 210 NSTEMI patients with de novo coronary lesions randomized to treatment with either a paclitaxel-coated DCB (SeQuent Please, B. Braun Melsungen AG, Germany) or a standard bare metal stent (BMS). DCB showed noninferiority to BMS since both the primary endpoint, target lesion failure, and the secondary endpoint, MACE, were comparable between the 2 groups (target lesion failure 3.8% vs 6.6% for DCB and BMS respectively, P=.53; MACE 6.7% vs 14.2% for DCB and BMS respectively, P=.11).18
In the prespecified subanalysis of the BASKET-SMALL 2 trial, which included lesions in vessels with diameter<3 mm, at 1-year follow-up there was no significant difference in the incidence of the primary endpoint, MACE, by randomized treatment in patients with ACS (HR, 0.50; 95%CI, 0.19-1.26 for DCB vs DES) or CCS (HR, 1.29; 95%CI, 0.67-2.47) for DCB vs DES and there was no interaction between clinical presentation and treatment effect (P for interaction .088).21
Nevertheless, a class effect for DCB is not to be expected given the huge heterogeneity in formulations, doses, release kinetics and drug-tissue interaction. Antiproliferative drugs belonging to the limus family usually have a worse transfer rate to tissues compared with paclitaxel. To overcome these limitations, several technologies have been implemented to provide sirolimus delivery, including those using a phospholipid drug carrier, on which the Magic Touch platform is based.3
The present study shows for the first time the performance of a sirolimus-eluting balloon in the setting of acute coronary syndromes. By direct comparison between ACS and CCS patients, we show that there was no significant difference in procedural and 1-year outcomes in terms of angiographic success and TLR, thus supporting its use in both settings without distinction. The higher incidence of MACE observed in ACS patients was mainly driven by the occurrence of new spontaneous MI (not procedurally related). This result could be expected, regardless of the device used, since recurrences are not uncommon after ACS (in this series ACS patients also had a higher rate of previous MI among baseline characteristics).
In addition, our study is based on an all-comers prospective registry that includes both small and large vessels, de novo and ISR lesions, single vessel, and multiple vessels disease.
Despite such a heterogenous population, the overall outcomes at 12 months of follow-up after Magic Touch use in ACS patients were good and not different from those of SCB use in CCS patients; notably, in this all-comers registry, the rates of TLR and MACE in the cohort of patients with de novo lesions (1.9% and 5.9%, respectively) are comparable to those reported for paclitaxel-coated balloons used for selected populations in the previously mentioned trials (such as the 3% TLR rate in the REVELATION trial or the 6.7% MACE rate in the PEP-CAD NSTEMI trial). In addition, current generation DES used in the acute setting yield similar results on follow-up. For example, the TLR incidence was 2.7% for a biodegradable polymer sirolimus-eluting stent in the MASTER study (which compared a DES with a BMS), while in the BIOSTEMI trial, comparing a biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent, the rate was 2% and 3%, respectively.22,23
The apparently worse outcomes for the Magic Touch DCB used in the cohort with ISR are still in line with previous studies on paclitaxel balloons used to treat ISR, which showed a TLR rate of 10%-15% at 12 months.24,25 Notably, our results are independent of the indication for PCI (ACS vs CCS) and confirm that treatment strategies for ISR remain a challenge.
Finally, our study shows similar predilatation rates and DCB inflation time between ACS and CCS. Moreover, we observed that even the presence of thrombus (detected in 91 ACS patients and preventively addressed with aspiration in a small proportion of these, 18.7%) did not prevent operators from using a DCB strategy.
LimitationsThe main limitations of this study can be summarized as follows: a) despite being an all-comers registry, it was restricted to centers experienced with DCB use and could not therefore be truly representative of real-world practice with DCB; b) there was no core-lab and both lesion assessment at baseline and evaluation of the final procedural results were left to each operator, thus limiting standardization in image interpretation; c) this is a post-hoc analysis and, although the CCS and ACS populations were numerically well balanced, our results should be considered hypothesis generating; similarly, ACS represents a heterogenous group in which ST-elevation MI, non–ST-elevation MI and unstable angina coexist but may respond differently to treatment. Accordingly, further studies and randomized trials are needed to confirm our observations and draw solid conclusions.
CONCLUSIONsThe Magic Touch sirolimus DCB shows good performance in terms of acute and 1-year outcomes whether used for PCI during ACS or CCS. In addition, despite often being time-consuming, a DCB strategy is feasible even in the acute setting where operators usually feel more comfortable with quicker and rapidly resolutive procedures.
- -
Limited data are available on the use of drug-coated balloons in acute coronary syndromes.
- -
To date, only paclitaxel-based drug-eluting platforms have been validated in this setting.
- -
For the first a time, a sirolimus-based platform (the Magic Touch balloon) showed equally good performance in terms of acute and 1-year outcomes whether used in acute or chronic coronary syndromes.
- -
Similar predilatation rates and inflation times between the acute and chronic settings suggest that longer procedural times, as expected when using a drug-coated balloon, do not prevent operators from applying this strategy even in the acute setting.
The original EASTBOURNE study was an independent, investigator-driven study that received funding from Envision Scientific; this society played no role in the protocol definition, selection of centers, conduct of the study, or interpretation of the results. The PEACE substudy did not receive any funding.
ETHICAL CONSIDERATIONSThe EASTBOURNE study received the approval of the central ethics committee of the coordinating center; data from the PEACE substudy are derived from the main registry. Each patient enrolled in the EASTBOURNE registry signed an informed consent that was archived. The sex variable is reported in accordance with the SAGER guidelines, and no impact on outcomes was expected. The STROBE form was completed.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence has been used in the preparation of this work.
AUTHORS’ CONTRIBUTIONSAll authors equally contributed to the study conception and design, data collection, analysis and interpretation, and manuscript preparation.
CONFLICTS OF INTERESTNone to disclose.