The use of the jailed guidewire technique is highly useful when treating bifurcation lesions by provisional stenting. However, at the time of withdrawal, the guidewire can suffer damage and even fracture. The aim of this study was to evaluate structural damage in both polymer-coated and nonpolymer-coated jailed guidewires.
MethodsBetween January 2011 and December 2012, an observational study was conducted using stereoscopic microscopy to evaluate 135 jailed guidewires (45 nonpolymer-coated and 90 polymer-coated) previously used in the percutaneous treatment of bifurcation lesions. Damage after withdrawal was classified as mild, moderate, or severe.
ResultsAge and sex distributions were similar in both groups of patients treated with polymer-coated and nonpolymer-coated guidewires. However, operators selected polymer-coated guidewires more frequently when treating more complex bifurcations and in diabetic patients. Some type of microscopic damage was observed in 25 of the guidewires analyzed (18%). Paradoxically, damage was more common in nonpolymer-coated guidewires (53.0% vs 1.1%; P<.001). None of the guidewires suffered complete fracture.
ConclusionsCoronary guidewires that are jailed during the treatment of bifurcations using provisional stenting often suffer nonsevere microscopic damage. Although polymer-coated guidewires were used in more complex bifurcation lesions, paradoxically, they were damaged less frequently.
Keywords
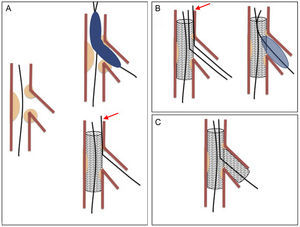
The treatment of coronary bifurcation lesions accounts for 15% to 20% of all percutaneous interventions.1 These lesions form part of the group of complex lesions and their treatment poses a major technical challenge. They have been associated with high rates of restenosis and complications, although these rates have been reduced by the use of new strategies. Simple or “provisional stenting” is the most widely accepted technique,2–5 which consists in stenting the main vessel (MV) and covering the origin of the side branch (SB). The SB can be predilated before stent implantation and the procedure concluded if good results are obtained (Figure 1A). If the outcome of the procedure is suboptimal, the SB can be postdilated through the metal structure of the stent and, again, the procedure can be concluded if successful (Figure 1B). Postdilation can be performed by dilation of the SB alone, sequential dilation of the SB and MV, or simultaneous dilation of both vessels (kissing-balloon technique). Stenting of the SB is reserved for patients with a suboptimal outcome after the above-mentioned maneuvers have been attempted (T-stenting) (Figure 1C). However, this strategy is not without risk because the SB can become occluded when a stent is implanted in the MV. Recrossing the SB can sometimes be difficult or impossible. This maneuver is facilitated by the “jailed guidewire technique”, which involves leaving a coronary guidewire in the SB jailed between the vessel wall and the stent struts. If the branch becomes occluded, this guidewire marks the position to facilitate access.6 However, there are several reports of severe complications caused by the guidewire fracturing during withdrawal.7–15
Simple or provisional stenting. A: stent implanted in the main vessel; the side-branch guidewire is jailed (arrow) between the main vessel wall and the stent body. B: recrossing and postdilation of the side branch; the jailed guidewire is withdrawn after introducing the balloon in the side branch. C: stent implantation in the side branch.
A pilot study was conducted using stereoscopic microscopy to evaluate damage to polymer-coated and nonpolymer-coated guidewires after they were jailed during percutaneous procedures using the jailed guidewire technique.
METHODSPatientsDuring 2011 and 2012, the study included 135 patients with bifurcation lesions treated with the provisional stenting technique. The patients met the following inclusion criteria: a) irrespective of its length, morphology or angulation, MV lesion > 50% located at a bifurcation point; b) MV diameter ≥ 2.5mm; c) SB diameter ≥ 2.25mm; and d) length of the stenosis in the SB < 10mm. Exclusion criteria: a) contraindications to dual antiplatelet therapy; b) acute phase of myocardial infarction (direct or rescue angioplasty), and c) cardiogenic shock. All patients gave written informed consent.
ProcedureAll interventions were performed using the femoral approach. All patients were treated using a stepwise provisional stenting strategy.16 A first stent was implanted in the MV and a guidewire was jailed between the stent mesh and the SB vessel wall. At this point, the SB ostium was evaluated. If the SB was compromised, another guidewire was advanced into the SB, the jailed guidewire was extracted, and the SB was simultaneously or sequentially dilated. After this maneuver, the SB ostium was again evaluated and a second stent was implanted in the SB at the discretion of the operator. The operator also chose the type of guidewire to be jailed. Two types were available: “nonpolymer-coated” BMW [Balance MiddleWeight] and Floppy II models; Abbot Vascular, Illinois, United States) and “polymer-coated” (Pilot 50 or Whisper MS models; Abbott Vascular). Procedural success was defined as TIMI (Thrombolysis In Myocardial Infarction) grade 3 flow in both the MV and SB and residual stenosis visually estimated as ≤ 20% in the MV. At the time of percutaneous coronary intervention, all patients were receiving dual antiplatelet therapy with acetylsalicylic acid and clopidogrel. Procedural anticoagulation was achieved with unfractionated heparin (100 U/kg intravenous bolus). Glycoprotein IIb/IIIa inhibitors were administered at the discretion of the operator. After the intervention, all patients received combined anticoagulant and dual antiplatelet therapy for 12 months and were instructed to continue aspirin indefinitely. After percutaneous coronary intervention, all patients underwent an electrocardiogram and troponin and creatine levels were measured at 6hours and 24hours. If clinically indicated, further electrocardiograms and enzyme tests were ordered. Non-Q-wave myocardial infarction was defined as an increase in the creatine kinase level up to 3 times the upper limit of the normal range.
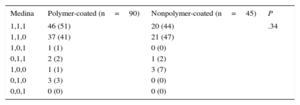
Angiographic StudyThe Medina classification was used to assess the anatomy of the bifurcation at baseline.17 All patients underwent angiograms before and after percutaneous coronary intervention, and the MV and SB reference diameters, minimal lumen diameters, and percentages of stenosis were measured. Data were obtained on a group of variables that could influence subsequent damage to the guidewire, such as the type of bifurcation, tortuosity, and calcification.
Microscopic StudyDuring the study period, coronary guidewires were consecutively collected that had been used to treat the bifurcation lesions using the jailed guidewire technique. After the guidewires were withdrawn, they were cleaned with an aqueous solution and allowed to dry before analysis. The microscopic study was performed using an SMZ-800 stereomicroscope (Nikon Instruments, Inc.; Melville, New York, United States). This microscope is mounted with a parallel double lens and interchangeable objectives with a magnification range of 1.0× to 6.3× and a standard visual field of 3.5 mm to 22.0 mm. Microphotographs were acquired using a DS-Fi1 color camera (two-thirds inch, high-density CCD; 5.24 million pixels; Nikon Instruments). Recorded image sizes range from 2560×1920 pixels (maximum 10 frames per second) to 1280×960 pixels (maximum 21 frames per second). A 100-W P-ICI2 coaxial episcopic illuminator (Nikon Instruments) was used to improve image quality. This device controls power and light intensity via an external transformer coupled by optical fiber.
Low magnification was used to evaluate the first 40cm of the distal end of the coronary guidewire and higher magnification was used to examine areas with suspected damage in more detail. The external and internal covers were examined for changes. Images were acquired of damage, which was graded into 5 categories:
- 1.
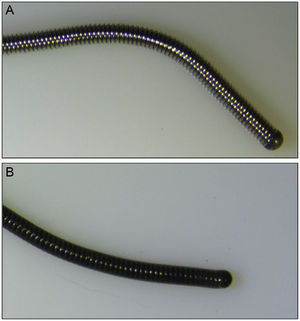
No damage: the guidewire suffered no loss of integrity over its entire length (Figure 2).
- 2.
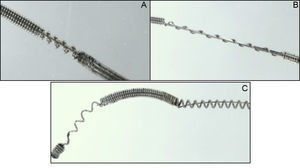
Slight damage (Figure 3A): the external cover suffered loss of integrity ≤ 2mm.
- 3.
Moderate damage (Figure 3B): the external cover suffered loss of integrity > 2mm.
- 4.
Severe damage (Figure 3C): visible changes to the inner cover of the guidewire.
- 5.
Fracture: discontinuity at some point along the guidewire.
Dichotomous variables are expressed as numbers and percentages of the total. Quantitative variables are expressed as mean±standard deviation. The chi-square test or Fisher's exact test was used to analyze differences between proportions. The Student-Fisher t test for unpaired data was used to compare continuous variables in both groups of patients. A P value of < .05 was used as a cutoff for statistical significance. All analyzes were performed using the SPSS 20 0.0 software package.
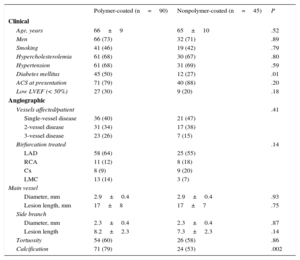
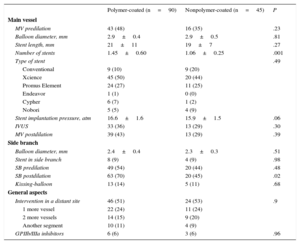
RESULTSBaseline DataTable 1 shows the baseline clinical and angiographic data. Most patients were admitted for acute coronary syndrome. The distributions by age, sex, risk factors, and clinical characteristics were similar in both groups (Table 1) except for that of diabetes mellitus, which was significantly higher in the polymer-coated guidewire group (50% vs 27%; P<.01). The most frequently affected bifurcation was the anterior descending artery/diagonal branch without significant differences between groups (Table 2). Table 3 shows the characteristics of the interventions. Lesions in both groups were treated in a similar manner. However, in the polymer-coated stent group significantly more stents were needed (1.45±0.60 vs 1.06±0.25 stents; P<.001) and the use of SB postdilation was significantly higher (70% vs 45%; P=.02). A second stent was rarely used in the SB and its use was almost identical in both groups (9%). There were no significant differences between groups regarding other technical aspects or the use of glycoprotein IIb/IIIa platelet inhibitors. Both groups were similar regarding bifurcation location, MV size, SB size, stenosis severity, and the type of bifurcation lesion according to the Medina classification (Tables 2 and 4). The prevalence of coronary calcification was significantly higher in the polymer-coated guidewire group (79% vs 53%; P<.002).
Baseline Data
| Polymer-coated (n=90) | Nonpolymer-coated (n=45) | P | |
|---|---|---|---|
| Clinical | |||
| Age, years | 66±9 | 65±10 | .52 |
| Men | 66 (73) | 32 (71) | .89 |
| Smoking | 41 (46) | 19 (42) | .79 |
| Hypercholesterolemia | 61 (68) | 30 (67) | .80 |
| Hypertension | 61 (68) | 31 (69) | .59 |
| Diabetes mellitus | 45 (50) | 12 (27) | .01 |
| ACS at presentation | 71 (79) | 40 (88) | .20 |
| Low LVEF (< 50%) | 27 (30) | 9 (20) | .18 |
| Angiographic | |||
| Vessels affected/patient | .41 | ||
| Single-vessel disease | 36 (40) | 21 (47) | |
| 2-vessel disease | 31 (34) | 17 (38) | |
| 3-vessel disease | 23 (26) | 7 (15) | |
| Birfurcation treated | .14 | ||
| LAD | 58 (64) | 25 (55) | |
| RCA | 11 (12) | 8 (18) | |
| Cx | 8 (9) | 9 (20) | |
| LMC | 13 (14) | 3 (7) | |
| Main vessel | |||
| Diameter, mm | 2.9±0.4 | 2.9±0.4 | .93 |
| Lesion length, mm | 17±8 | 17±7 | .75 |
| Side branch | |||
| Diameter, mm | 2.3±0.4 | 2.3±0.4 | .87 |
| Lesion length | 8.2±2.3 | 7.3±2.3 | .14 |
| Tortuosity | 54 (60) | 26 (58) | .86 |
| Calcification | 71 (79) | 24 (53) | .002 |
ACS, acute coronary syndrome; Cx, circumflex artery; LAD, left anterior descending artery; LMC, left main coronary artery; LVEF, left ventricular ejection fraction; RCA, right coronary artery.
Values are expressed as no. (%) or mean±standard deviation.
Intervention Data
| Polymer-coated (n=90) | Nonpolymer-coated (n=45) | P | |
|---|---|---|---|
| Main vessel | |||
| MV predilation | 43 (48) | 16 (35) | .23 |
| Balloon diameter, mm | 2.9±0.4 | 2.9±0.5 | .81 |
| Stent length, mm | 21±11 | 19±7 | .27 |
| Number of stents | 1.45±0.60 | 1.06±0.25 | .001 |
| Type of stent | .49 | ||
| Conventional | 9 (10) | 9 (20) | |
| Xcience | 45 (50) | 20 (44) | |
| Promus Element | 24 (27) | 11 (25) | |
| Endeavor | 1 (1) | 0 (0) | |
| Cypher | 6 (7) | 1 (2) | |
| Nobori | 5 (5) | 4 (9) | |
| Stent implantation pressure, atm | 16.6±1.6 | 15.9±1.5 | .06 |
| IVUS | 33 (36) | 13 (29) | .30 |
| MV postdilation | 39 (43) | 13 (29) | .39 |
| Side branch | |||
| Balloon diameter, mm | 2.4±0.4 | 2.3±0.3 | .51 |
| Stent in side branch | 8 (9) | 4 (9) | .98 |
| SB predilation | 49 (54) | 20 (44) | .48 |
| SB postdilation | 63 (70) | 20 (45) | .02 |
| Kissing-balloon | 13 (14) | 5 (11) | .68 |
| General aspects | |||
| Intervention in a distant site | 46 (51) | 24 (53) | .9 |
| 1 more vessel | 22 (24) | 11 (24) | |
| 2 more vessels | 14 (15) | 9 (20) | |
| Another segment | 10 (11) | 4 (9) | |
| GPIIb/IIIa inhibitors | 6 (6) | 3 (6) | .96 |
GPIIb/IIIa, glycoprotein IIb/IIIa inhibitors; IVUS, intravascular ultrasound; MV, main vessel; SB, side branch.
Values are expressed as no. (%) or mean±standard deviation.
Quantitative Angiographic Study
| Polymer-coated (n=93) | Nonpolymer-coated (n=45) | P | |
|---|---|---|---|
| Minimal luminal diameter, mm | |||
| Main vessel | |||
| Baseline | 0.59±0.3 | 0.52±0.3 | .26 |
| Postintervention | 2.8±0.4 | 2.8±0.4 | .81 |
| Side branch | |||
| Baseline | 1.04±0.7 | 1.70±0.8 | .002 |
| Postintervention | 1.9±0.6 | 1.9±0.7 | .93 |
| Stenosis, % | |||
| Main vessel | |||
| Baseline | 80±10 | 82±9 | .26 |
| Postintervention | 5±4 | 4±3 | .47 |
| Side branch | |||
| Baseline | 53±32 | 31±31 | .003 |
| Postintervention | 17±19 | 12±18 | .19 |
Values are expressed as mean ± standard deviation.
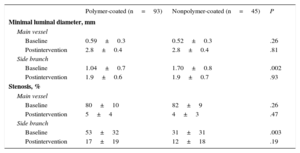
Table 4 shows the quantitative angiographic data. The minimal lumen diameter, percentage of stenosis in the treated segments, and the immediate postintervention reduction in this percentage was similar in the MV of both groups. However, lesions in the origin of the SB were more severe in the polymer-coated guidewire group; the percentage of stenosis in the origin of the SB was higher (53%±32% vs 31%±31%; P<.003) and the minimum luminal diameter was smaller (1.04±0.7mm vs 1.70±0.8mm; P<.002). There were no significant differences between the 2 types of guidewires regarding the development of complications. There was 1 death in each group. One patient in the nonpolymer-coated guidewire group died of ventricular fibrillation 48hours after the procedure, and 1 patient in the polymer-coated guidewire group died after a compassionate revascularization procedure.
Results of Microscopic AnalysisSome type of microscopic damage was observed in 25 of the guidewires analyzed (18%). Damage was more frequent in nonpolymer-coated guidewires (53.0% vs 1.1%; P<.001). However, severe damage was observed in only 2 guidewires (1.5%), both of which were nonpolymer-coated. There were no cases of complete fracture. Table 5 shows the results of the microscopic analysis of the guidewires.
DISCUSSIONThis study showed that during the treatment of bifurcations using stents, 18% of jailed coronary guidewires suffered some type of microscopic damage, but with no clinical repercussions. Damage was more common in nonpolymer-coated guidewires.
Currently, provisional stenting is the preferred strategy for the percutaneous treatment of coronary bifurcations.2–5 The jailed guidewire technique is recommended when applying this strategy, especially when complex bifurcations are being treated.18
Advantages of Using the Jailed GuidewireThe implantation of a stent in the MV can displace the carina, which may affect or occlude the origin of the SB. In these situations, the jailed guidewire helps to keep the SB open and, in the event of occlusion, the guidewire is the only marker of its position. It also facilitates access to the SB by favorably changing the angle of the bifurcation. An additional advantage is that it anchors the guidewire, thus facilitating the intubation of the guidewire catheter and providing firmer support for the balloon to cross the origin of the SB. Finally, in extreme situations, it can be used to introduce a low-profile balloon and dilate the SB, and to conclude the intervention using the inverted crush technique or to redilate the stent crushed in the MV once the branch occlusion has been resolved.19
Risks of the Jailed Guidewire TechniqueGiven that the MV stent is retained under pressure against the arterial wall, the guidewire jailed between these 2 structures may suffer damage and even fracture at the time of withdrawal. Although rare, the guidewire can fracture during withdrawal, which is a severe procedural complication that may require its urgent surgical removal.7–15 However, preventative measures exist, which include avoiding the following: the use of polymer-coated guidewires, locating the distal portion of the guidewire in small-caliber secondary vessels, using high pressures during stent release, covering a large portion of the jailed guidewire with stents, and postdilation of the MV with balloon, which should only be done if needed and only with short “noncompliant” balloons.9
Currently, a wide variety of coronary guidewires are available with different properties that make them suitable for various indications. According to their external coating, guidewires can be classified as polymer-coated or nonpolymer-coated (Figure 2). Although there are no randomized studies that have determined the most suitable guidewire in the jailed guidewire technique, expert recommendations advise against the use of polymer-coated guidewires during this technique based on the argument that that they are more susceptible to fracture than nonpolymer-coated guidewires.6,8,20 Given that the fracture rate is very low, it would be difficult to design a randomized study to identify which type of guidewire is more resistant to fracture during this technique.
This pilot study used microscopic damage to the guidewire as a surrogate variable of fracture. It was found that a non-negligible percentage of nonpolymer-coated guidewires suffered some type of external damage and even inner damage, whereas only 1 polymer-coated guidewire showed moderate damage to the external coating (Figure 4). Microscopic damage was not associated with clinical events and there were no fractures. The use of polymer-coated guidewires was not associated with an increased number of complications. Thus, the relevance of this study lies in its technical aspects, rather than in its clinical aspects.
LimitationsThis study was an observational study. In addition, the choice of guidewire was left to the operator's discretion, once the coronary anatomy and characteristics of the bifurcation had been assessed. The operators chose polymer-coated guidewires for more complex, more calcified, and more severe bifurcations. Although this situation may have led to negative bias regarding the polymer-coated guidewire group, paradoxically these guidewires suffered the least damage (despite their not being recommended for this use).
The study also has 3 methodological limitations. Firstly, the choice of only 2 types of guidewire per group could hinder the extrapolation of the results to other types of guidewires. Secondly, a traditional stereomicroscope was used to evaluate damage. The optical resolution of this instrument is limited, which we sought to overcome by the use of a coaxial episcopic illuminator. Thirdly, the proposed classification for assessing the damage was arbitrary and the use of > 2mm in length as a cutoff point for damage was chosen on the basis of our own observations that the use of a shorter length makes it difficult to identify damage by visual inspection. It would be of little relevance to identify only minor damage by the use of sophisticated technology.
Since no articles have been published on the present topic, we believe that the results of this study can provide a basis for future research using larger randomized trials.
CONCLUSIONSCoronary jailed guidewires used in the treatment of bifurcations through provisional stenting often suffer nonsevere microscopic damage. Although the polymer-coated and nonpolymer-coated guidewires were equally effective in accessing the SB, they were more frequently damaged when jailed after stent implantation in the MV.
FUNDINGThis project was funded under the Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica 2008-2011, ISCIII (Instituto de Salud Carlos III), PI12/00440, cofunded by FEDER (Fondo Europeo de Desarrollo Regional).
CONFLICTS OF INTERESTM. Pan receives small fees from Abbott Vascular for presenting conferences.