Dual antiplatelet therapy (DAPT), defined as the addition of an oral P2Y12 receptor inhibitor to aspirin, is necessary in all patients undergoing percutaneous coronary intervention (PCI). This universal recommendation stems from clinical trials showing that, compared with aspirin, DAPT significantly lowers risk for both short- and long-term thrombotic events. Current guidelines favor a 6-month DAPT duration for patients with stable symptoms and 12 months after acute coronary syndrome (ACS).1 However, the salutary benefits of DAPT occur at the expense of bleeding, which accrues in a gradual fashion with ongoing exposure to platelet inhibition. Classically perceived as a nuisance complication of antithrombotic therapy, prevention of bleeding has emerged as an important clinical priority, a therapeutic shift that is attributable to several reasons. First, post-PCI hemorrhage is associated with a large and durable risk for mortality that is comparable to that of myocardial infarction (MI).2 While the links between intracranial hemorrhage and hemodynamically significant bleeds and near-term mortality are self-evident, less severe bleeds requiring blood transfusion or hospitalization also increase mortality risk. Second, contemporary drug eluting stent platforms are characterized by thin struts and biocompatible polymers, features that lower device thrombogenicity and alter the risk-benefit calculus for extended DAPT durations.3 Third, high bleeding risk (HBR) conditions such as renal impairment, active malignancy and advanced age, are increasingly prevalent among PCI patients, rendering the toxicity of DAPT vis a vis bleeding more pronounced relative to its putative benefits. As a result of these developments, novel therapeutic approaches for post-PCI antithrombotic therapy consider the prevention of both ischemic and bleeding events as equivalent goals. One strategy is to shorten the duration of DAPT with an early transition to antiplatelet monotherapy with either aspirin or a P2Y12 inhibitor.
EXPERIMENTAL BASISAspirin exerts a relatively modest platelet inhibitory effect compared with the oral P2Y12 inhibitors clopidogrel, prasugrel, and ticagrelor. Hence, the addition of a P2Y12 inhibitor to aspirin yields a synergistic effect on platelet inhibition and accounts for the superiority of DAPT vs aspirin alone with respect to thrombotic risk reduction. However, several lines of experimental and clinical evidence support the opposite—namely that P2Y12 inhibition alone is sufficient to mitigate ischemic events while avoiding aspirin-related bleeding. In a report involving healthy volunteers, Armstrong et al.4 found that the platelet inhibitory effects of aspirin were minimal in the presence of strong P2Y12 blockade. Ex vivo studies in higher-risk patients are consistent with these results. For example, the pharmacodynamic effect of ticagrelor alone was comparable to ticagrelor plus aspirin with respect to thrombus generation among high-risk patients undergoing PCI.5 These experimental results are supported by findings from clinical trials showing the safety of aspirin withdrawal while maintaining P2Y12 inhibition with ticagrelor.6
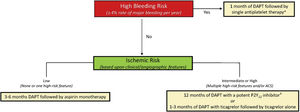
BLEEDING AND ISCHEMIC RISK FRAMEWORKUse of antiplatelet monotherapy, as with any antithrombotic strategy, should be tailored to the patient's unique bleeding and thrombotic risk profile. Accordingly, evaluating risk for both types of events is a key initial step to inform subsequent clinical decisions. However, clinical assessment of ischemic and bleeding risk is challenging given the substantial overlap in underlying conditions that contribute to either complication (ie, renal impairment, older age, active malignancy). In this regard, the introduction of a standardized classification for HBR, proposed by the Academic Research Consortium (BARC), is an important development for both clinicians and investigators.7 Within this construct, HBR is defined as an annualized risk for major bleeding of at least 4%, and patients fulfilling at least 1 major or 2 minor criteria may be categorized as HBR (table 1). Clinical trials have examined different antithrombotic strategies, including antiplatelet monotherapy, across the spectrum of bleeding and thrombotic risk. Within this framework, clinical decisions with respect to duration and intensity of platelet inhibition may occur in a sequential fashion with consideration of bleeding followed by ischemic risk (figure 1). As discussed below, HBR patients may be further categorized in relation to the presence of absence of atrial fibrillation (AF). Among non-HBR patients, consideration of thrombotic risk (stable vs acute) may then inform decisions surrounding DAPT.8
Academic Research Consortium high bleeding risk criteria*.
| Major | Minor |
|---|---|
| Long-term anticoagulation | Age ≥ 75 years |
| eGFR <30 mL/min/1.73 m2 | eGFR 30–59 mL/min/1.73 m2 |
| Hemoglobin <11 g/dL | Hemoglobin 11-12.9 g/dL for men and11–11.9 g/dL for women |
| Spontaneous bleeding in prior 6 mo | Spontaneous bleeding requiring hospitalization or transfusion in the past 12 mo |
| Platelet count <100 x 109/L | Long-term use of oral NSAIDs or steroids |
| Chronic bleeding diathesis | |
| Liver cirrhosis with portal hypertension | |
| Active malignancy within past 12 mo | |
| Spontaneous ICH; traumatic ICH in prior 12 mo; brain arteriovenous malformation; moderate or severe ischemic stroke in prior 6 mo | Any ischemic stroke not meeting the major criterion |
| Nondeferrable major surgery on DAPT | |
| Major surgery or major trauma within 30 d of PCI |
Framework for duration and intensity of dual antiplatelet therapy in relation to bleeding and ischemic risk. High bleeding risk is defined by the Academic Research Consortium as a major bleeding rate of at least 4% at 1 year or intracranial hemorrhage risk of 1% at 1 year. Ischemic risk is categorized based on clinical (diabetes mellitus requiring medication, peripheral arterial disease, acute coronary syndrome) and angiographic (multivessel percutaneous coronary intervention; stent length> 60mm; calcification requiring atherectomy) features associated with excess thrombosis. ACS, acute coronary syndrome. DAPT, dual antiplatelet therapy. Adapted, with permission from Baber et al.8
">a High bleeding risk patients with concomitant atrial fibrillation receiving oral anticoagulation may be treated with clopidogrel alone and a direct oral anticoagulant.
">b Deescalation from prasugrel or ticagrelor to clopidogrel may be considered based upon genetic or platelet function testing.
Use of triple therapy, defined as DAPT plus an oral anticoagulant (OAC), may be required among patients with AF undergoing PCI but results in a prohibitive risk for bleeding. Hence, minimizing the duration of triple therapy has emerged as a key priority in the setting of HBR patients. An aspirin-free strategy was first examined in such patients in a modest-sized randomized trial comparing dual pathway inhibition (DPI), consisting of a vitamin K antagonist (VKA) plus clopidogrel, vs conventional triple therapy.9 Over 1 year, the primary endpoint of major or minor bleeding was reduced by an absolute 40% with the experimental intervention. Importantly, aspirin withdrawal did not result in an incremental risk for MI or death. This initial observation has been extended and confirmed in larger trials wherein DPI is achieved with a direct oral anticoagulant (DOAC) and antiplatelet monotherapy, usually clopidogrel. The benefits of DPI, compared with VKA-based triple therapy, are attributable to both aspirin withdrawal and DOAC use based on insights from the AUGUSTUS trial.10 In this large randomized study, which used a 2 x 2 factorial design, patients with AF undergoing PCI or with ACS were randomized to VKA vs DOAC and also aspirin vs placebo. Over a median follow-up of 6 months, clinically significant bleeding was reduced by 40% and 20% among those allocated to placebo (vs aspirin) and DOAC (vs VKA), respectively. Similar findings favoring an overall benefit with DOAC-based DPI have been shown in other trials enrolling similar patient cohorts. As a result of this accumulated evidence base, consensus recommendations favor a default strategy of an OAC plus a single antiplatelet agent in most patients with AF undergoing PCI.11 In most trials, aspirin was administered at the time of PCI and usually until hospital discharge. Hence the timing of aspirin withdrawal and, by extension, commencement of dual therapy, is generally at the time of discharge following PCI. Nonetheless, certain patients at particularly high thrombotic risk may benefit from a slightly longer duration of DAPT and therefore receive triple therapy for up to 1 month after PCI. Accordingly, contemporary consensus favors a short duration of triple therapy in patients at high thrombotic and low bleeding risk. After 1 year of DPI, antiplatelet therapy may be discontinued and patients can be maintained on an OAC alone.
HBR without AFNotwithstanding the bleeding risk related to AF and triple therapy, other commonly encountered conditions including renal impairment, anemia and older age also serve as HBR criteria. In such patients, there remains an important clinical need to minimize the duration of DAPT and lower bleeding risk as much as possible. While current guidelines recommend a 3-month DAPT duration following PCI with drug eluting stents in the setting of HBR, several clinical trials demonstrate equipoise for durations as short as 1 month. The LEADERS FREE trial examined the safety and efficacy of a bare metal vs drug-coated stent among patients with at least 1 HBR criteria (n=2466).12 The mean age of the study population was 75 years and approximately 25% presented with a troponin (+) ACS. The study protocol stipulated all patients receive 4 weeks of DAPT followed by antiplatelet monotherapy with a preference for aspirin. Despite a very short DAPT duration, the 1-year rate of BARC type 3 or 5 bleeding was over 7%, easily exceeding the 4% threshold set by the HBR-ARC. In a separate study, the safety and efficacy of a short (28-day) vs longer (6-month) DAPT duration was examined in an HBR cohort treated with the cobalt-chromium everolimus-eluting stent. Between months 1 and 6, the primary endpoint of all-cause death or MI occurred less frequently among patients receiving 1 month of DAPT (3.5% vs 4.3%; Pnon-inferiority<.0005).13 BARC type 3 or 5 bleeding was also reduced by over 50% (2.2% vs 4.5%; P=.016). In aggregate, multiple studies have examined the impact of antiplatelet monotherapy across the spectrum of HBR patients undergoing PCI. In the presence of AF, DAPT may be required until hospital discharge after which time most patients can be treated with a single antiplatelet agent (preferably clopidogrel) and an OAC. In non-AF HBR patients, a very short DAPT duration of 4 weeks followed by antiplatelet monotherapy (preferably aspirin) is supported by randomized evidence.
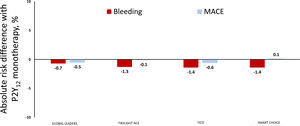
Non-HBR: ACSIn the setting of ACS, potent P2Y12 inhibition with ticagrelor or prasugrel is superior to clopidogrel in preventing thrombotic events, albeit at an excess cost of bleeding. Aspirin withdrawal followed by P2Y12 inhibitor monotherapy may allow ACS patients to experience the benefits of strong platelet inhibition while avoiding aspirin-related toxicity, as summarized in table 2 and figure 2. Thus, extension of the “aspirin-free” paradigm to non-AF PCI patients was first examined in the GLOBAL LEADERS trial, which compared 4 weeks of DAPT with aspirin and ticagrelor followed by ticagrelor monotherapy for 23 months vs a conventional antiplatelet strategy among 15?968 PCI patients.18 While the primary endpoint of all-cause death or Q-wave MI was nonsignificantly different between groups at 2 years (3.8% vs 4.4%; P=.07), a post hoc analysis involving ACS participants suggested a significant reduction in BARC type 3 or 5 bleeding at 1 year among those receiving ticagrelor alone (0.8% vs 1.5%; P=.004).14 Concordant results were observed in a prespecified analysis of non–ST-segment elevation ACS patients randomized in the TWILIGHT trial.15 Ticagrelor monotherapy, compared with ticagrelor plus aspirin, resulted in a 64% reduction in BARC type 3 or 5 bleeding while rates of all-cause death, MI or stroke were comparable between groups (4.3% vs 4.4%; P=.84). A similarly designed randomized trial that enrolled ACS patients exclusively (36% with ST-segment elevation MI) reported comparable reductions in major bleeding without incremental thrombotic risk with ticagrelor monotherapy.16 Although experimental studies suggest a limited pharcodynamic effect of aspirin in the setting of P2Y12 inhibition with prasugrel, an aspirin-free approach with prasugrel monotherapy after PCI remains untested in a large, adequately powered randomized trial. Other studies have examined clopidogrel monotherapy vs DAPT with aspirin plus clopidogrel among relatively low-risk patients after 1 to 3 months of DAPT.17,19 While these studies suggest significant reductions in bleeding without an ischemic penalty with use of clopidogrel alone, pharmacodynamic variability in clopidogrel response and CYP2C19 genetic polymorphisms that impact clopidogrel metabolism render such an approach difficult to implement as a default strategy. Moreover, more potent P2Y12 inhibitors are preferentially recommended to clopidogrel among ACS patients undergoing PCI. Hence, pending further data, the clearest evidence supporting the safety and efficacy of an aspirin-free strategy in non-AF PCI patients is based upon ticagrelor monotherapy in the setting of ACS.
Clinical trials examining aspirin withdrawal in acute coronary syndrome
| Trial | P2Y12 | Sample size | Timing of aspirin withdrawal | Major bleeding rate (DAPT arm), % |
|---|---|---|---|---|
| GLOBAL LEADERS (ACS), Tomaniak et al.14 | Ticagrelor | 7487 | 1 mo | 1.5 |
| TWILIGHT-ACS, Baber et al.15 | Ticagrelor | 4614 | 3 mo | 2.1 |
| TICO, Kim et al.16 | Ticagrelor | 3056 | 3 mo | 2.9 |
| SMART CHOICE (ACS), Hahn et al.17 | Clopidogrel | 1741 | 3 mo | 1.0 |
ACS, acute coronary syndrome; DAPT, dual antiplatelet therapy.
Aspirin withdrawal in acute coronary syndromes. Y-axis depicts absolute risk difference in rates of major bleeding (red bars) and major adverse cardiovascular events (MACE; blue bars) among patients with ACS receiving P2Y12 inhibitor monotherapy vs dual antiplatelet therapy in select clinical trials. Negative values indicate lower risk with P2Y12 inhibitor monotherapy while positive values indicate the opposite; bleeding defined as Bleeding Academic Research Consortium type 3 or 5 in GLOBAL LEADERS14 and TWILIGHT-ACS15; Thrombolysis in Myocardial Infarction Major in TICO16 and BARC types 2 to 5 in SMART-CHOICE17.
Patients with stable ischemic heart disease undergoing PCI comprise a relatively low-risk cohort with respect to both thrombotic and hemorrhagic events. Thus, the clinical rationale for therapeutic strategies to lower bleeding (ie, aspirin withdrawal) or reduce ischemic risk (ie, potent P2Y12 inhibition) are less apparent among such patients relative to their higher-risk counterparts. In support of this hypothesis, the effect of ticagrelor monotherapy on reducing BARC type 2, 3 or 5 bleeding was attenuated among patients with stable vs acute syndromes in the TWILIGHT trial (Pint=.03).15 Several studies have examined the impact of short- (3-6 months) vs 12-month DAPT durations in low-risk patients undergoing PCI. In general, these studies did not find any incremental benefit to extending DAPT beyond 6 months while bleeding risk was numerically increased. These results support contemporary guidelines recommending 6 months of DAPT with clopidogrel in most low-risk stable patients undergoing PCI with drug eluting stents. Hence, the central justification for antiplatelet monotherapy varies somewhat by clinical phenotype: avoiding DAPT-related toxicity for HBR patients and lack of incremental benefit with prolonging DAPT among low-risk patients with stable ischemic heart disease.
CONCLUSIONAntiplatelet monotherapy following a brief duration of DAPT has emerged as a viable therapeutic strategy across a spectrum of patients undergoing PCI with an established and growing evidence base. The application of this approach depends on the patient's risk for bleeding and thrombosis. While HBR patients with AF may be treated with an OAC and a P2Y12 inhibitor immediately following PCI, non-AF HBR patients may require a short (up to 4 weeks) course of DAPT followed by antiplatelet monotherapy. For non-HBR patients with ACS, ticagrelor monotherapy provides the benefits of strong platelet inhibition while avoiding aspirin-related bleeding. For non-HBR patients with stable symptoms, a short duration of DAPT followed by aspirin monotherapy provides adequate protection against ischemic risk compared with longer DAPT durations.
FUNDINGThis work received no funding.
CONFLICTS OF INTERESTU. Baber declares having received honoraria from AstraZeneca and Amgen.