To the Editor:
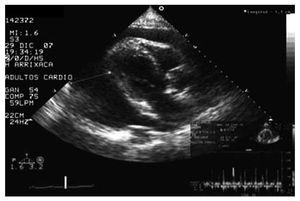
The case of an 80 year old male is presented. Type 2 diabetic, recently diagnosed with latent multiple myeloma and a history of severe right coronary lesion, revascularised through angioplasty and stent implant 2 years before. The echocardiography performed 2 years previously presented normal global and segmental contractility of the left ventricle (LV) and mild chronic pericardial effusion (maximum diameter, 8 mm). The patient attended the emergency unit with intense asthenia and maintained hypotension over the previous week. In the physical exam, the patient presented a blood pressure of 90/40 mm Hg, heart rate 120 beats/ min, raised jugular venous pressure, lower limb oedema, and paradoxical pulse. The electrocardiogram displayed diffuse low voltage QRS and the chest x-ray, general cardiomegaly. With these findings, an emergency echocardiogram was performed (Figure), which confirmed the clinical suspicion of cardiac tamponade showing severe and diffuse pericardial effusion (maximum diameter, 53 mm), paradoxical movement in the interventricular septum, right atrial collapse and transmitral flow variation >25% with respiration. An emergency pericardiocentesis was performed, with immediate drainage of 1200 mL of serous-sanguineous fluid and rapid improvement of the clinical and haemodynamic parameters. In the immediate echocardiographic monitoring, the normality of the diameters was checked and the LV systolic function (end diastolic diameter, 47 mm; LV ejection fraction of the LVEF >60%), with mild residual persistent pericardial effusion (maximum diameter, 11 mm). The cytologic, biochemical, microbiological, and immunological studies did not identify specific effusion aetiology.
Figure.Echocardiograph image showing cardiac tamponade from severe pericardial effusion.
Two days after the pericardiocentesis, the patient presented rapidly progressing dyspnea, with clinical symptomatology of pulmonary oedema and signs of low cardiac output. The emergency echocardiogram displayed severe global contractile dysfunction of the LV (LVEF, 13%). The ECG and myocardial enzymes obtained sequentially did not display data of acute ischemia. The patient required treatment with intravenous furosemide and inotropic support with intravenous dobutamine and dopamine, which were then phased out. Contractility of the LV normalised on the sixth day (LVEF, 64%). A coronary catheterisation was performed which displayed a lesion in the posterolateral branch of the right coronary artery, did not justify the symptoms and was not revascularised.
Transitory left ventricular dysfunction after pericardiocentesis is a rare complication and its exact incidence is unknown. Its chronology is variable and it can appear from hours1-4 to several days after the procedure, with development of pulmonary oedema and even cardiogenic shock. In the described cases, such as ours, ventricular function gradually normalised in 7 to 10 days, with complete recovery and good mid-term prognosis.1-6 The first to discover it were Vandyke et al2 in 1983, and attributed the symptoms to a sudden increase in venous return when vascular endurance was still high, when a discrepancy between preload and afterload is produced. Afterwards, other authors observed new cases and proposed other hypotheses: haemodynamic such as in Konstam et al,6 based on greater volume overload in the right ventricle compared to the left after decompression of the pericardium; and neuroendocrine, attributed to the symptomatology of an overstimulation of the sympathetic nervous system.3 Other authors have pointed out that the dysfunction could be due to the decrease in coronary flow from pericardial fluid compression on the epicardiac coronary arteries,7 and ischaemic cardiopathy could be a favouring factor. In the cases published to date, a greater prevalence of severe neoplastic effusion has been observed, which indicates that this could also be a favouring factor.1-3
Therefore, although it is an infrequent complication, the transitory dysfunction of the LV after pericardiocentesis should be known and taken into account to assure correct clinical handling. A more progressive drainage and more intense follow-up, during and after the procedure, could help to prevent and identify this complication in the early stages.