The Berlin-Heart EXCOR ventricular assist device (Berlin-Heart AG, Germany) is a paracorporeal pulsed flow device driven by a pneumatic mechanism. Currently, the main application is as a bridge to heart transplant, both in adults and children.1,2
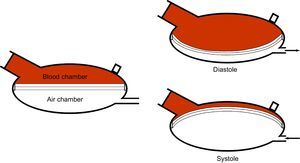
The device comprises a pneumatic driving unit, a system of tubes, and a ventricle or pump. This pump is divided into 2 chambers (blood chamber and air chamber) by a 3-layer polyurethane membrane. The negative and positive pressure in diastole and systole, respectively, generated by the drive unit and transmitted to the ventricle, displace the membrane, with the corresponding effect of suction or blood expulsion (Figure 1). Mechanical problems occur more frequently in pulsed flow devices than in continuous flow ones.3 Membrane rupture is, however, an uncommon problem and has been reported in a limited number of cases,4–6 after more than 2000 implants up until 2015. We present 2 cases of membrane rupture in our center.
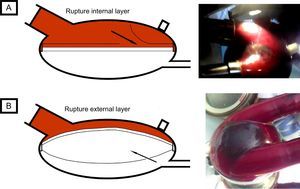
The first case occurred in a 62-year-old man, with a biventricular Berlin-Heart EXCOR implant after acute myocardial infarction complicated with cardiogenic shock as a bridge to heart transplant. On day 77 of treatment, while the patient was on the ward, a mobile shadow, measuring 3cm across, was detected in the right pump, with an irregular border and fine bands of adhered fibrin. This image was apparent in systole and disappeared in diastole (Figure 2A and ). It was only visible in the blood chamber; the membrane appeared normal from the air chamber. The patient was asymptomatic and hemodynamically stable, with no warning alarms showing on the display of the operating panel. Membrane rupture was suspected and an emergency pump change was undertaken. After explantation, a torn area was detected on the internal layer of the membrane (adjacent to the blood chamber).
The second case occurred in a 63-year-old man with dilated cardiomyopathy, who had been implanted with a Berlin-Heart EXCOR device as a bridge to heart transplant. On day 161 of assist, while on the ward, the patient suddenly experienced dyspnea, malaise, and cold sweat. The physical examination confirmed cardiogenic shock, with a warning of “low left ventricular flow” showing on the display of the operating panel. A perfectly round shadow measuring 5cm in diameter was detected in the blood chamber. This shadow was more evident during device systole (Figure 2B and ). In the air chamber, a rounded bulge was observed in systole, while the appearance was normal during diastole. Both the shadow and bulge became increasingly marked in the course of minutes. Membrane rupture was suspected and an emergency left pump change was undertaken. After explantation, a small tear (measuring a few millimeters) could be seen on the outer layer of the membrane (adjacent to the air chamber) and air was trapped between the external and middle layer.
Both cases illustrate 2 different presentations of membrane rupture. To date, cases of rupture of the internal and external layer of the membrane have been described (with no reports of rupture of the middle layer).
Rupture of the internal layer (first patient) meant that the blood came into contact with the nonbiocompatible surfaces between the internal and middle layer, thereby favoring the formation of fibrin and thrombi in this area. The middle and external layers remained intact and pump function was maintained. As the tear spread, a flap could be detected in the blood chamber. This appeared and disappeared during the device cycle, with an irregular border and chaotic movement (Figure 2A). By contrast, the appearance from the air chamber was normal. This type of rupture, therefore, increases the risk of thrombosis, and it is hemodynamically tolerated because the assist device continues to function correctly.
Rupture of the outermost layer (second case) led to progressive air entrapment between the middle and external layer: the air entered in the space during systole, and its exit in diastole was hindered by the valve effect generated by the suction of the device (Figure 2B). External examination revealed a rounded shadow in the blood chamber during systole, whereas a rounded bulge was seen in the air chamber during systole. This mechanism of air entrapment led to progressive pump dysfunction (pump obstruction) that had a substantial hemodynamic impact on the patient. In this situation, cardiogenic shock can develop in a question of minutes.
The 2 cases presented and the schematics help us to understand the development of this complication, each one with a different appearance on examination, different clinical course, and different associated complications. In our opinion, awareness of this complication and its pathophysiology is important for early detection, as emergency replacement of the ventricle is essential to avoid adverse outcomes.