The plaque distribution patterns in coronary bifurcation lesions are not well understood. It has been speculated that carina is free of plaque partly because of high wall shear stress providing an atheroprotective effect. The objective was to study plaque distribution with intravascular ultrasound (IVUS) in the coronary bifurcation and the prevalence of carina involvement.
MethodsIVUS study was performed on 195 coronary bifurcation lesions in the main vessel (MV) and on 91 in the side branch (SB). Plaque at the carina was considered when its thickness was >0.3mm. Plaque burden was measured at different levels: proximal reference, distal, carina and at the point of minimal lumen area (MLA).
ResultsThe prevalence of plaque at the carina was 32%. Its thickness was 0.8 (0.36) mm, less than that observed at the counter-carina [1.22 (0.54) mm; P<.01]. The prevalence was higher (52%) when the MLA point was distal to the carina. The plaque at the carina was associated with a lower incidence of damage at the SB ostium after stenting the MV (32% versus 54%; P<.04).
ConclusionsThe carina is not immune to atherosclerosis, showing plaque at this level in one-third of the bifurcations. The incidence of plaque is higher in those bifurcations with the MLA point distal to the carina and seems to be associated with a lower incidence of damage to the SB ostium.
Keywords
Currently, the development patterns for atherosclerotic coronary artery disease are better understood in the non-bifurcated segments than in the coronary bifurcations.1,2 From an anatomical perspective, the coronary bifurcations consist of three components that come together at the carinal area: the proximal main vessel (MV), the distal main vessel and the side branch (SB), meaning that a classification is needed that defines whether the disease is present in each of these components.3,4 It is essential to take into account, however, that lesions in coronary bifurcations have additional attributes that are relevant from the perspective of endoluminal treatment, such as the spatial orientation of the SB with respect to the MV (bifurcation angle), the length of the SB and its importance (amount of myocardium irrigated) and the length of the lesion in the SB.5,6 Studying these lesions with intravascular ultrasound (IVUS), which provides precise anatomical information about plaque distribution and morphology of the carina visible in the longitudinal reconstruction of the IVUS,7,8,9 also allows us to observe the presence or absence of plaque at this unique part of the bifurcation.
Isolated observations of pathologist have reported the presence of plaque at the carina as unusual,10 while IVUS studies analyzing plaque distribution at lesions in bifurcations have not provided data about the prevalence of plaque at the carina.11,12,13 An atheroprotective mechanism has been reported in this component of the bifurcation as a consequence of high wall shear stress (HWSS),14,15 in contrast to low wall shear stress (LWSS) observed in the counter-carina. The assumption in the interventionist community that the carina is free of plaque is probably a product of these findings. From our perspective, however, atherogenesis is a complex, poorly understood and multifactorial phenomenon and it would be surprising if a single atheroprotective factor were sufficient to confer immunity to the carina.
The aim of this study (combining IVUS study of cross sections and longitudinal reconstruction) is to analyze the distribution of atheromatous plaque at lesions affecting a bifurcation and to determine the prevalence of plaque at the carina. In addition, in the subgroup of bifurcations without SB origin involvement5 (_,_,0), this study attempts to determine the possible relationship between the presence of plaque at the carina and damage to the SB ostium once the stent has been implanted in the MV.
Methods PatientsThe study included 185 consecutive patients with 195 de novo lesions in a coronary bifurcation. All lesions were treated percutaneously in the period spanning June 2007 to June 2009. Bifurcation lesions were excluded if the SB had a diameter <2.25mm. The clinical characteristics of the patients are summarized in Table 1. Most patients were male (74%) and the prevalence of diabetes mellitus was 29%. All patients underwent diagnostic cardiac catheterization. After the diagnostic phase and documentation of disease in a coronary bifurcation, an IVUS study was performed of the MV in all cases and of the SB in 91 out of 195 lesions (47%). The MV study was repeated after stent implantation.
Table 1. Clinical Characteristics of the Patients (n=185) *
| Age (years) | 61 (10) |
| Males | 145 (74) |
| Arterial hypertension | 119 (61) |
| Diabetes mellitus | 56 (29) |
| Dyslipidemia | 91 (48) |
| Active smoker | 81 (42) |
| Prior PCI | 12 (17) |
| Unstable situation | 121 (62) |
| Multivessel CAD (≥2 vessels) | 96 (49) |
| Ejection fraction (%) | 55 (9) |
Abbreviations: CAD, coronary artery disease; PCI, percutaneous coronary intervention.
* Data express n (%) or mean (standard deviation).
For the diagnostic cardiac catheterization, all patients underwent left ventriculography with a 30° right anterior oblique projection and coronary angiography with multiple projections. Filming used two orthogonal projections in all cases, to visualize the SB ostium as clearly as possible. Once the diagnostic study was complete, a 10,000 IU dose of heparin sodium was administered. After a guide wire was positioned in the MV and another in the SB, a baseline IVUS study was conducted. The drug-eluting stent was selected according to the reference diameters of the MV and implanted at 14–16atm. Once the stent was implanted, the two angiographies selected in the diagnostic phase were repeated to assess the state of the SB ostium. Further study of the MV with IVUS was then conducted.
Cases with documented stenosis ≥70% in the SB were treated with angioplasty and, if the attending operator felt it was necessary, a second stent was implanted. Once the procedure was complete, the heparin was neutralized with protamine sulfate16 and the femoral puncture was closed with a percutaneous closure device (Angioseal®). For angiographic measurements, including the bifurcation angle, we used the MEDIS CMS 7.1 dedicated system (The Netherlands). All measurements were made off-line by two expert interventional cardiologists.
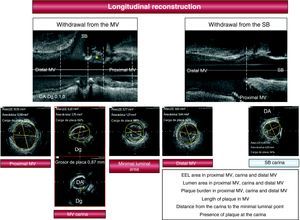
Intravascular Ultrasound StudyThe IVUS study protocol is illustrated in Figure 1. After the diagnostic coronary angiography, a bolus of intracoronary nitroglycerin was administered and a baseline ultrasound study of the MV was conducted (Atlantis SR, Boston Scientific, 2.5 F, 40MHz) with motorized withdrawal of 0.5mm/s from the distal reference segment. At the operator's discretion, a motorized withdrawal from the SB was also performed on 91 of the 195 lesions (47%). The area of the external elastic lamina, the luminal area and the plaque burden were determined at various points: proximal reference segment, carina, the minimal luminal point and distal reference segment. We also calculated the remodeling index (area of the external elastic lamina at the carina level/area of the external elastic lamina in the distal reference segment). With the longitudinal reconstruction of the IVUS (Figure 1), we analyzed the morphology of the carina and the distribution of the atheroma plaque at the bifurcation (in the proximal MV, carinal area, distal MV and SB) and also measured the distance from the carina to the minimal luminal point. When plaque was found at the carina, its thickness was measured in the corresponding cross section using a caliper included in the IVUS software. The carina was considered to have plaque if this atheroma plaque thickness was ≥0.3mm.
Figure 1. Ultrasound study protocol. The asterisk indicates plaque at the carina. Plaque burden=(EEL Area−lumen area)/EEL DA, descending artery; Dg, diagonal; EEL: external elastic lamina; MV, main vessel; SB, side branch.
The prevalence of plaque at the carina was analyzed in terms of: (a) the location of the bifurcation in the coronary tree (left main coronary artery [LMCA] or distal coronary bifurcations); (b) the location of the point of maximum stenosis in the MV, and (c) the type of bifurcation according to the Medina classification. After the stent was implanted in the MV, the IVUS study was repeated to observe the behavior of the carina.
Statistical AnalysisQuantitative variables are expressed as mean (standard deviation) and the qualitative data in percentages. To compare means, Student's t-test was used for independent data. To compare the qualitative variables, the χ2 test was used. Values of P<.05 were considered statistically significant. We used SPSS version 15.0 for statistical calculations.
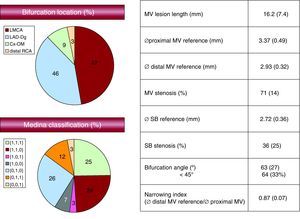
ResultsThe baseline angiographic findings are shown in Figure 2. The lesion was located in the LMCA in half of the cases, with the rest being distally located. The bifurcation between the anterior descending artery and the diagonal artery was the most prevalent location in this subgroup.
Figure 2. Baseline angiographic data. Cx-OM, circumflex artery-oblique marginal artery; LAD-Dg, anterior descending artery-diagonal artery; LMCA, left maincoronary artery; MV, main vessel; RCA, right coronary artery; SB, side branch.
Regarding the type of bifurcation, the SB was affected in 40% of the cases (type 1,1,1; 0,1,1; 1,0,1 lesions). The bifurcation angle was 63° (27°). The bifurcation angle was <45° in 64 of the 195 lesions (33%).
In all cases, a drug-eluting stent was implanted. For 80 lesions (41%), a stent was implanted in the MV without further manipulation at the SB ostium level. In 26 lesions (13%), a balloon angioplasty was performed in the SB after stent implantation in the MV. The procedure was completed with two balloons inflated simultaneously (kissing balloon technique) for 67 cases (34%). In 22 bifurcations (11%), a second stent had to be implanted in the SB (T-stent). No cases of acute or subacute stent thrombosis were found.
Quantitative IVUS data are detailed in Table 2. Plaque burden in the maximum stenosis segment was 73% (12%) and the ultrasonic length of the lesion was 18 (7) mm.
Table 2. Baseline Ultrasound Characteristics (n=195) *
| MV proximal reference | |
| EEL area (mm2) | 17.33 (5.14) |
| Luminal area (mm2) | 11.99 (4.61) |
| Plaque burden (%) | 31 (14) |
| Carina | |
| EEL area (mm2) | 11.06 (4.28) |
| Luminal area (mm2) | 4.32 (2.43) |
| Plaque burden (%) | 59 (17) |
| Presence of plaque at the carina | 32 (63/195) |
| Thickness of plaque at the carina (n=63) (mm) | 0.81 (0.36) |
| Thickness of plaque at the counter-carina (mm) | 1.22 (0.54) |
| MV distal reference | |
| EEL area (mm2) | 11.22 (4.27) |
| Luminal area (mm2) | 7.86 (3.18) |
| Plaque burden (%) | 28 (15) |
| Remodeling index (ostium) | 0.8 (0.21) |
| Minimal luminal area | |
| EEL area (mm2) | 11.83 (3.99) |
| Luminal area (mm2) | 3.04 (1.48) |
| Plaque burden (%) | 73 (12) |
| Distance to the carina (mm) | 3.27 (3.5) |
| Side branch ostium (n=91) | |
| EEL area (mm2) | 8.28 (4.37) |
| Luminal area (mm2) | 4.5 (2.63) |
| Plaque burden (%) | 42 (19) |
Abbreviations: EEL: external elastic lamina; MV: main vessel.
Data express n (%) or mean (standard deviation).
* Remodeling index=ostium EEL area/ EEL distal reference area.
The presence of plaque at the carina was documented in 32% of lesions (63 out of 195) with a thickness of 0.8 (0.36) mm. In most cases, the plaque thickness at the carinal level was less than that observed in the counter-carina (1.22 (0.54), P<.01), although the opposite was found for 10 of the 63 cases (16%).
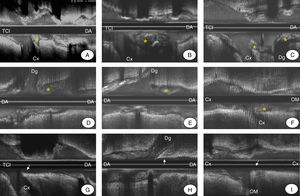
The prevalence of plaque at the carina of the LMCA was similar to that observed in bifurcations with a more distal location (33% versus 31%). Figure 3 shows various examples of bifurcation lesions in the LMCA and distal bifurcations with and without plaque at the carina.
Figure 3. Examples of intravascular ultrasound that show the presence of plaque at the carina (asterisk) at the left main coronary artery (LMCA) distal (A–C), at the anterior descending artery-diagonal artery (LAD-Dg) (D and E) and at the circumflex artery-oblique marginal artery (Cx-OM) (F). Examples are also provided of coronary bifurcations with no plaque at the carina (arrow) at the LMCA (G), at the LAD-Dg (H) and at the Cx-OM (I).
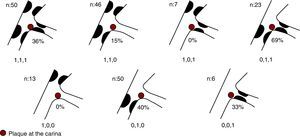
The difference in prevalence of plaque at the carina according to the type of bifurcation established in the Medina classification was 15% in 1,1,0 lesions and no plaque was observed in 1,0,0 and 1,0,1 lesions (Figure 4).
Figure 4. Prevalence of plaque at the carina according to bifurcation type.
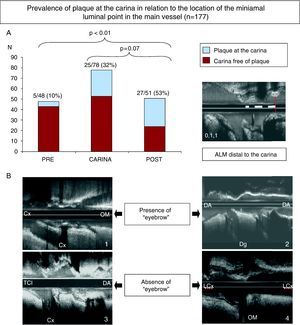
Lesions with the point of maximum stenosis (minimal luminal area) located distal to the carina had greater prevalence of plaque than the lesions in which the point of maximum stenosis was located at the carina level or proximal to the carina (Figure 5A).
Figure 5. (A) The graph shows the prevalence of plaque at the carina depending on the location of the point with minimal luminal area in the main vessel. Note that the prevalence of plaque was greater when the location of the minimal luminal area was distal to the carina. (B) Examples of bifurcation with eyebrow sign with plaque (1) and without plaque (2) at the carina. There are also examples with absence of eyebrow sign, with plaque (3) and without plaque (4) at the carina. MLA, minimal luminal area; Cx/LCx, circumflex artery; DA, anterior descending artery; Dg, diagonal artery; OM, oblique marginal artery; LMCA, left main coronary artery.
In coronary bifurcations whose carina showed a spiky ultrasonic morphology (“eyebrow sign”),7 the prevalence of plaque was less (27% versus 37%), although not statistically significant (Figure 5B).
With respect to the angiographic bifurcation angle, there was no difference in the prevalence of plaque at the carina between bifurcations with acute angles (≤45°) and the rest (33% versus 29%, respectively). The bifurcation angle was 61° (25°) with plaque at the carina and 65° (23°) without plaque (no significant differences).
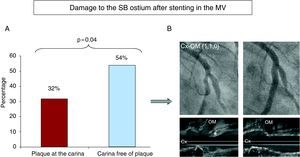
In the subgroup of bifurcation lesions with no disease in the SB (_,_,0), we analyzed the possible relationship between the presence of plaque at the carina and damage to the SB ostium (resulting angiographic stenosis ≥50%) after stent implantation in the MV. Damage to the SB ostium was less prevalent in lesions that had plaque at the carina (32% versus 54%, P=.04) (Figure 6).
Figure 6. (A) The graph shows the lower incidence of damage in the side branch ostium when there was plaque at the carina. (B) Example of bifurcations with plaque at the carina (asterisk) and the absence of damage in the side branch ostium after the stent was implanted in the main vessel. Cx, circumflex artery; OM, oblique marginal artery.
The ultrasound examination showed that the mechanism of ostial SB damage after stent implantation in the MV was always due to displacement of the carina. No cases showed plaque shifting towards the SB ostium (“snow-plow” phenomenon).17
DiscussionPatterns of atherosclerotic disease development continue to be poorly understood, especially in coronary bifurcations.1,2 Recently, based on post-mortem anatomical observations, the interventionist community has considered the presence of plaque at the carina of the bifurcation as unusual.10 Our study shows that the carina is not immune to developing atherosclerosis and that a third of the coronary bifurcation lesions that are treated in clinical practice show atheroma plaque at this level. This discrepancy can be explained by the design characteristics of our ultrasound study, performed on a consecutive series of bifurcation lesions treated percutaneously, as opposed to post-mortem observations, which obviously cannot represent clinical reality.
In contrast to the results obtained in our study, Oviedo et al.18 recently published a study with IVUS on 126 LMCA bifurcations, with motorized withdrawal from the MV and from the SB, in which they conclude that the carina is free of plaque. Perhaps these discrepancies can be explained by considering that the studies use different methodologies. Oviedo et al. studied the distribution of plaque at the carinal level by analyzing cross sections, where our study analyzed both the cross sectional axis and longitudinal axis, which unequivocally determined the presence or absence of plaque at the carina (Figure 3). Van der Giessen et al.15 published similar results on the prevalence of plaque at the carina in a study using computed angiotomography, a technique with lower resolution than IVUS for studying this phenomenon, and in a series with less cases.
Our findings are also consistent with ultrasound observations reported by Shimada et al.13 who, in cases where there was plaque at the carina, observed a plaque thickness less than that found in the counter-carina.
The similarity between the behavior of the lesions located in the LMCA and the distribution of atheroma plaque is remarkable, especially the similar prevalence of plaque at the carina and at the distal bifurcations of the coronary tree. Also noteworthy is the limited influence that the angiographic angle seems to have on the formation of plaque at the carinal level.
Regarding type 1,1,0 bifurcation lesions, a low prevalence of plaque at the carina and even absence of plaque in 1,0,0 and 1,0,1 lesions were also found, which is difficult to explain with the data available.
We consider important that the presence of plaque at the carina was greater in the bifurcations that had maximum stenosis located distal to the carina in the MV. It could be argued that a stenosis distal to the carina could alter the flow velocity patterns and promote plaque development by reducing the atheroprotective effect, transforming a HWSS region into a LWSS region.
Recently, we reported a peculiar spiky morphology at the carina (“eyebrow sign”)7 in ostial lesions of the LAD, making treatment with a provisional stent vulnerable due to its shifting, which affects the ostium of the circumflex artery (Cx). The presence of this morphological pattern of the carina is associated with lower prevalence of plaque in our study, although it is not significant.
When considering to what extent the presence of plaque at the carina may be involved in inducing damage in the SB ostium after implanting a stent in the MV, we observed, paradoxically, less damage to the SB ostium when there is plaque at the carina. This could be because the plaque makes the carina more resistant, limiting the carina shifting that is the dominant mechanism causing damage to the SB ostium when a stent is implanted.7 It is interesting to note that during IVUS examination after stent implantation in the MV we observed no cases of plaque shift at the carina towards the SB ostium.
There are still no randomized studies that show the benefits of IVUS in treating bifurcation lesions. Many studies use IVUS to define the quality of scaffolding after stent treatment, given that it is widely recognized as a useful tool. We believe that adding a baseline IVUS evaluation of lesions that affect a coronary bifurcation provides qualitative and quantitative information of great interest that can enrich the data provided by angiography and could inform treatment strategies. Ideally, IVUS studies should be performed on both bifurcation vessels. Oviedo et al. published a study on LMCA bifurcations that showed the limitations of the SB ostium assessment when carried out at the expense of information obtained with the withdrawal from the MV.19
Limitations of the StudyOne significant limitation of this study is that the number of bifurcations studied is relatively small for analyzing such a complex phenomenon as the presence or absence of plaque at the carina, given that several bifurcation types found in various locations were studied. We therefore believe it would be very useful to conduct a study of a greater number of bifurcation lesions. Another limitation of this study was that we did not have access to SB baseline ultrasound information for half of the patients.
ConclusionsOur ultrasound study of coronary bifurcations lesions showed a prevalence of plaque at the carina of 32%. The prevalence appears to be greater when the point of maximum stenosis in the MV is located distal to the carina, which may be related to gradual reduction of the atheroprotective effect that HWSS provides to the carina.
From the therapeutic standpoint, stent implantation in the MV and the presence of plaque at the carina seem to have a protective effect for the SB ostium. This could be because plaque makes the carina more resistant, limiting carina shift when the stent is implanted.
Conflicts of interestThe authors state that they have no conflicts of interest.
Acknowledgements
We would like to thank Charina Medina for helping us to edit and proofread this text.
Received 12 April 2010
Accepted 26 July 2010
Corresponding author. Servicio de Cardiología, Hospital Universitario de Gran Canaria Dr. Negrín, Barranco de la Ballena, s/n. 35010 Las Palmas de Gran Canaria, Spain. pemarlor@hotmail.com