We aimed to describe the clinical outcomes of the use of the CentriMag acute circulatory support system as a bridge to emergency heart transplantation (HTx).
MethodsWe conducted a descriptive analysis of the clinical outcomes of consecutive HTx candidates included in a multicenter retrospective registry who were treated with the CentriMag device, configured either for left ventricular support (LVS) or biventricular support (BVS). All patients were listed for high-priority HTx. The study assessed the period 2010 to 2020 and involved 16 transplant centers around Spain. We excluded patients treated with isolated right ventricular support or venoarterial extracorporeal membrane oxygenation without LVS. The primary endpoint was 1-year post-HTx survival.
ResultsThe study population comprised 213 emergency HTx candidates bridged on CentriMag LVS and 145 on CentriMag BVS. Overall, 303 (84.6%) patients received a transplant and 53 (14.8%) died without having an organ donor during the index hospitalization. Median time on the device was 15 days, with 66 (18.6%) patients being supported for> 30 days. One-year posttransplant survival was 77.6%. Univariable and multivariable analyses showed no statistically significant differences in pre- or post-HTx survival in patients managed with BVS vs LVS. Patients managed with BVS had higher rates of bleeding, need for transfusion, hemolysis and renal failure than patients managed with LVS, while the latter group showed a higher incidence of ischemic stroke.
ConclusionsIn a setting of candidate prioritization with short waiting list times, bridging to HTx with the CentriMag system was feasible and resulted in acceptable on-support and posttransplant outcomes.
Keywords
Recent changes in organ donor allocation policies have led to an increasing use of temporary mechanical circulatory support for bridging patients to heart transplantation (HTx),1 as a result of the prioritization of these candidates on the waiting list.2 Currently, approximately 30 to 50 of all HTx performed annually in Spain correspond to candidates bridged with temporary devices,3 including intra-aortic balloon pumps, percutaneous ventricular assist devices (VAD), extracorporeal membrane oxygenation (ECMO), and surgically implanted, nondischargeable extracorporeal VAD.4 Within this latter group, the Abbot CentriMag (Abbott, United States) system is particularly popular.
The Abbot CentriMag acute circulatory support system (formerly known as Levitronix CentriMag or Thoratec CentriMag) uses a magnetically levitated centrifugal pump than can provide up to 10 liters per minute of blood flow under optimal loading conditions.5 The system was approved for providing up to 30 days of extracorporeal left ventricular support (LVS), right ventricular support, or biventricular support (BVS) to hospitalized patients; however, good outcomes have been reported even with longer periods of use.6 This pump may also be used as part of ECMO circuits, eventually combined with LVS.7 The device is mostly implanted as a bridge to cardiac recovery or, if not possible, to a definitive therapeutic decision, such as HTx or durable VAD implantation.
Full median sternotomy and direct cannulation of the great thoracic vessels and heart chambers constitute the classic surgical approach to implanting an extracorporeal CentriMag circuit. However, less invasive techniques can be used. LVS can be provided by means of an inflow cannula inserted into the left ventricle or atrium through lateral thoracotomy, combined with an outflow cannula inserted in the subclavian/axillary or femoral artery8,9; chest incisions can be avoided by guiding the inflow cannula to the left atrium through the interatrial septum from a transfemoral venous access.10 For right ventricular support, the pulmonary artery can be cannulated through a single transjugular venous access.11 It is still not known whether less invasive approaches for CentriMag implantation may result in a clinical benefit compared with classic implantation.
The aim of our study was to describe the clinical outcomes of patients bridged to HTx with the CentriMag system configured for LVS or BVS. For this purpose, we analyzed the clinical information collected in a retrospective multi-institutional Spanish database.4
METHODSStudy descriptionThe ASIS-TC study was a retrospective registry conducted in 16 Spanish HTx centers.4,12 This registry included consecutive adult patients listed for emergency HTx while being supported with intra-aortic balloon pumps, extracorporeal membrane oxygenation, percutaneous VADs or surgically implanted, nondischargeable extracorporeal VADs from January 1, 2010 to December 31, 2020. We excluded patients listed for a second HTx or multi-organ transplantation. The research project was approved by the Ethics Committee for Clinical Research of the Autonomous Community of Galicia (Spain). Informed consent was waived given the retrospective study design.
For the present analysis, we selected from the ASIS-TC study database all patients who were listed for emergency HTx while being treated with an extracorporeal circulatory support system that used a CentriMag pump and was configured for providing LVS or BVS We excluded patients managed with isolated right ventricular support or venoarterial ECMO without LVS.
According to the Spanish organ allocation protocol, patients bridged to HTx on CentriMag LVS or BVS were given the maximum level of waiting list priority, known as “status 0”, throughout the study period.4 Status 0 implied priority for getting the first suitable heart donor retrieved within the whole of Spain. Specific criteria for waiting list prioritization in Spain during the study period are detailed in .
Follow-up and study outcomesPatients were followed up for 1 year after HTx or, if they were discharged alive without undergoing HTx, for 1 year after hospital discharge. Vital status at the end of follow-up was known in all patients.
We analyzed the incidence of relevant clinical outcomes of circulatory support, including HTx, death without HTx, hospital discharge without HTx, and transition to other modes of mechanical circulatory support. We also assessed the incidence of adverse clinical events associated with mechanical circulatory support, such as infection, device malfunction, bleeding, thromboembolism, stroke, cardiac reoperation, hemolysis, and renal failure, as well as in-hospital postoperative outcomes following HTx. Specific definitions of the study outcomes are detailed in the supplementary data. Clinical outcomes were allocated by local investigators.
Statistical analysisContinuous variables are presented as means± standard deviation or, when markedly asymmetric, as medians [nterquartile range]. Categorical variables are presented as proportions. Statistical comparisons were conducted by means of the chi-square test, the student t test, and the Mann-Whitney U test, as appropriate.
Multivariable logistic regression was used to control for the effect of potential confounders in the association between the type of support (BVS vs LVS) and the risk of death without HTx. In view of the number of events (n=53), we entered a maximum of 5 covariables in the model, selected on the basis of previous literature, clinical knowledge, and their asymmetric distribution between the 2 study groups, age, ischemic heart disease, previous sternotomies, temporary mechanical circulatory support before CentriMag implantation, and Interagency Registry for Mechanically Assisted Circulatory Support status.
One-year posttransplant survival was assessed by means of the Kaplan-Meier method. Group comparisons were performed by means of the log-rank test. Multivariable Cox regression was used to control for the effect of potential confounders on the association between the type of support (BVS vs LVS) and 1-year posttransplant mortality. In view of the number of events (n=68), we entered a maximum of 7 covariables in the model, selected on the basis of previous literature, clinical knowledge, and their asymmetric distribution between study groups, age, ischemic heart disease, previous sternotomies, Interagency Registry for Mechanically Assisted Circulatory Support status, diabetes, chronic obstructive pulmonary disease, and malignancy.
We also conducted an exploratory comparison of pre- and posttransplant outcomes in patients who underwent device implantation through full median sternotomy vs less invasive approaches.
Statistical analyses were performed with SPSS 25. Statistical significance was set as P <.05.
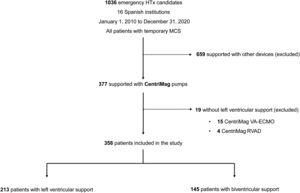
RESULTSPatients and devicesThe ASIS-TC registry included 1036 patients on the waiting list for emergency HTx in 16 Spanish institutions from 2010 to 2020. Among them, 377 patients were managed with extracorporeal circulatory support systems that used the CentriMag pump. We excluded 15 patients managed with venoarterial ECMO with no LVS and 4 patients managed with right ventricular support. Therefore, the study population was composed of 358 patients. A flow chart detailing the selection process of the study population is shown in figure 1.
CentriMag was used for LVS in 213 (59.5%) patients and for BVS in 145 (40.5%) patients. The BVS circuit included an oxygenator in 9 patients.
Devices were implanted through full median sternotomy in 277 (77.4%) patients, and less invasively in 81 (22.6%) patients: through thoracotomies in 79 patients and without chest incisions in 2 patients (transeptal approach). A systemic inflow cannula was placed in the left ventricle in 222 (62%) patients and in the left atrium in 136 (38%) patients. Arterial outflow was cannulated centrally, through the thoracic aorta, in 292 (81.6%) patients and peripherally in 66 (18.4%) patients: in the subclavian/axillary artery 60 (16.7%) and in the femoral artery in 6 (1.7%). A distal arterial cannula for anterograde limb perfusion was inserted in 3 patients who underwent peripheral arterial cannulation.
Among 145 patients who required biventricular support, a venous inflow cannula was inserted directly to the right atrium/thoracic cava vein in 136 (93.8%) and was guided to the same site through transfemoral venous access in 9 (6.2%). A pulmonary outflow cannula was inserted directly into the pulmonary artery in 144 (99.3%) patient and was guided to the same anatomical location through transjugular venous access in 1 (0.7%).
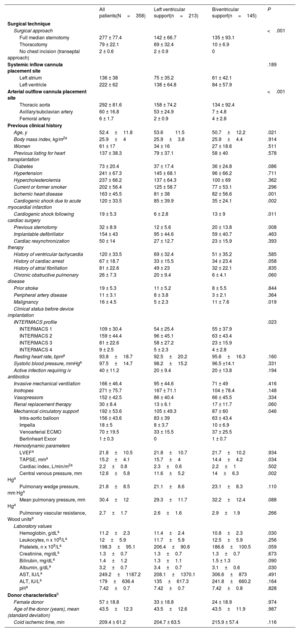
Baseline clinical characteristicsTable 1 shows the baseline clinical characteristics of study patients before device implantation.
Baseline clinical characteristics and clinical status of study patients before CentriMag implantation according to the type of support
| All patients(N=358) | Left ventricular support(n=213) | Biventricular support(n=145) | P | |
|---|---|---|---|---|
| Surgical technique | ||||
| Surgical approach | <.001 | |||
| Full median sternotomy | 277 ± 77.4 | 142 ± 66.7 | 135 ± 93.1 | |
| Thoracotomy | 79 ± 22.1 | 69 ± 32.4 | 10 ± 6.9 | |
| No chest incision (transeptal approach) | 2 ± 0.6 | 2 ± 0.9 | 0 | |
| Systemic inflow cannula placement site | .189 | |||
| Left atrium | 136 ± 38 | 75 ± 35.2 | 61 ± 42.1 | |
| Left ventricle | 222 ± 62 | 138 ± 64.8 | 84 ± 57.9 | |
| Arterial outflow cannula placement site | <.001 | |||
| Thoracic aorta | 292 ± 81.6 | 158 ± 74.2 | 134 ± 92.4 | |
| Axillary/subclavian artery | 60 ± 16.8 | 53 ± 24.9 | 7 ± 4.8 | |
| Femoral artery | 6 ± 1.7 | 2 ± 0.9 | 4 ± 2.8 | |
| Previous clinical history | ||||
| Age, y | 52.4±11.8 | 53.611.5 | 50.7±12.2 | .021 |
| Body mass index, kg/m2a | 25.9±4 | 25.9±3.8 | 25.9±4.4 | .914 |
| Women | 61 ± 17 | 34 ± 16 | 27 ± 18.6 | .511 |
| Previous listing for heart transplantation | 137 ± 38.3 | 79 ± 37.1 | 58 ± 40 | .578 |
| Diabetes | 73 ± 20.4 | 37 ± 17.4 | 36 ± 24.8 | .086 |
| Hypertension | 241 ± 67.3 | 145 ± 68.1 | 96 ± 66.2 | .711 |
| Hypercholesterolemia | 237 ± 66.2 | 137 ± 64.3 | 100 ± 69 | .362 |
| Current or former smoker | 202 ± 56.4 | 125 ± 58.7 | 77 ± 53.1 | .296 |
| Ischemic heart disease | 163 ± 45.5 | 81 ± 38 | 82 ± 56.6 | .001 |
| Cardiogenic shock due to acute myocardial infarction | 120 ± 33.5 | 85 ± 39.9 | 35 ± 24.1 | .002 |
| Cardiogenic shock following cardiac surgery | 19 ± 5.3 | 6 ± 2.8 | 13 ± 9 | .011 |
| Previous sternotomy | 32 ± 8.9 | 12 ± 5.6 | 20 ± 13.8 | .008 |
| Implantable defibrillator | 154 ± 43 | 95 ± 44.6 | 59 ± 40.7 | .463 |
| Cardiac resynchronization therapy | 50 ± 14 | 27 ± 12.7 | 23 ± 15.9 | .393 |
| History of ventricular tachycardia | 120 ± 33.5 | 69 ± 32.4 | 51 ± 35.2 | .585 |
| History of cardiac arrest | 67 ± 18.7 | 33 ± 15.5 | 34 ± 23.4 | .058 |
| History of atrial fibrillation | 81 ± 22.6 | 49 ± 23 | 32 ± 22.1 | .835 |
| Chronic obstructive pulmonary disease | 26 ± 7.3 | 20 ± 9.4 | 6 ± 4.1 | .060 |
| Prior stroke | 19 ± 5.3 | 11 ± 5.2 | 8 ± 5.5 | .844 |
| Peripheral artery disease | 11 ± 3.1 | 8 ± 3.8 | 3 ± 2.1 | .364 |
| Malignancy | 16 ± 4.5 | 5 ± 2.3 | 11 ± 7.6 | .019 |
| Clinical status before device implantation | ||||
| INTERMACS profile | .023 | |||
| INTERMACS 1 | 109 ± 30.4 | 54 ± 25.4 | 55 ± 37.9 | |
| INTERMACS 2 | 159 ± 44.4 | 96 ± 45.1 | 63 ± 43.4 | |
| INTERMACS 3 | 81 ± 22.6 | 58 ± 27.2 | 23 ± 15.9 | |
| INTERMACS 4 | 9 ± 2.5 | 5 ± 2.3 | 4 ± 2.8 | |
| Resting heart rate, bpma | 93.8±18.7 | 92.5±20.2 | 95.6±16.3 | .160 |
| Systolic blood pressure, mmHga | 97.5±14.7 | 98.2±15.2 | 96.5 ±14.1 | .331 |
| Active infection requiring iv antibiotics | 40 ± 11.2 | 20 ± 9.4 | 20 ± 13.8 | .194 |
| Invasive mechanical ventilation | 166 ± 46.4 | 95 ± 44.6 | 71 ± 49 | .416 |
| Inotropes | 271 ± 75.7 | 167 ± 71.1 | 104 ± 78.4 | .148 |
| Vasopressors | 152 ± 42.5 | 86 ± 40.4 | 66 ± 45.5 | .334 |
| Renal replacement therapy | 30 ± 8.4 | 13 ± 6.1 | 17 ± 11.7 | .060 |
| Mechanical circulatory support | 192 ± 53.6 | 105 ± 49.3 | 87 ± 60 | .046 |
| Intra-aortic balloon | 156 ± 43.6 | 83 ± 39 | 63 ± 43.4 | |
| Impella | 18 ± 5 | 8 ± 3.7 | 10 ± 6.9 | |
| Venoarterial ECMO | 70 ± 19.5 | 33 ± 15.5 | 37 ± 25.5 | |
| Berlinheart Excor | 1 ± 0.3 | 0 | 1 ± 0.7 | |
| Hemodynamic parameters | ||||
| LVEFa | 21.8±10.5 | 21.8±10.7 | 21.7±10.2 | .934 |
| TAPSE, mma | 15.2±4.1 | 15.7±4 | 14.4±4.2 | .034 |
| Cardiac index, L/min/m2a | 2.2±0.8 | 2.3±0.6 | 2.2±1 | .502 |
| Central venous pressure, mm Hga | 12.6±5.8 | 11.6±5.2 | 14±6.3 | .002 |
| Pulmonary wedge pressure, mm Hga | 21.8±8.5 | 21.1±8.6 | 23.1±8.3 | .110 |
| Mean pulmonary pressure, mm Hga | 30.4±12 | 29.3±11.7 | 32.2±12.4 | .088 |
| Pulmonary vascular resistance, Wood unitsa | 2.7±1.7 | 2.6±1.6 | 2.9±1.9 | .266 |
| Laboratory values | ||||
| Hemoglobin, g/dLa | 11.2±2.3 | 11.4±2.4 | 10.8±2.3 | .030 |
| Leukocytes, n x 109/La | 12±5.9 | 11.7±5.9 | 12.5±5.9 | .256 |
| Platelets, n x 109/La | 198.3±95.1 | 206.4±90.6 | 186.6±100.5 | .059 |
| Creatinine, mg/dLa | 1.3±0.7 | 1.3±0.7 | 1.3±0.7 | .673 |
| Bilirubin, mg/dLa | 1.4±1.2 | 1.3±1.1 | 1.5 ± 1.3 | .090 |
| Albumin, g/dLa | 3.2±0.7 | 3.4±0.7 | 3.1±0.6 | .030 |
| AST, IU/La | 249.2±1187.2 | 208.1±1370.1 | 306.6±873 | .491 |
| ALT, IU/La | 179±636.4 | 135±617.3 | 241.8±660.2 | .164 |
| pHa | 7.42±0.7 | 7.42±0.7 | 7.42±0.8 | .828 |
| Donor characteristicsb | ||||
| Female donor | 57 ± 18.8 | 33 ± 18.8 | 24 ± 18.9 | .974 |
| Age of the donor (years), mean (standard deviation) | 43.5±12.3 | 43.5±12.6 | 43.5±11.9 | .987 |
| Cold ischemic time, min | 209.4 ± 61.2 | 204.7 ± 63.5 | 215.9 ± 57.4 | .116 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECMO, extracorporeal membrane oxygenation; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annulus plane systolic excursion.
The data are presented as No. (%), mean±standard deviation, or median [interquartile range].
Missing values: resting heart rate (n=75), systolic blood pressure (n=79), body mass index (n=38), left ventricular ejection fraction (n=16), tricuspid annulus plane systolic excursion (n=173), cardiac index (n=169), central venous pressure (n=123), pulmonary wedged pressure (n=154), mean pulmonary pressure (n=131), pulmonary vascular resistance (n=189), hemoglobin (n=14), leucocytes (n=16), platelets (n=19), creatinine (n=14), bilirubin (n=77), albumin (n=190), AST (n=73), ALT (n=67), pH (n=83).
Patients managed with LVS were older and had a higher prevalence of ischemic heart disease and cardiogenic shock related to myocardial infarction than patients managed with BVS. Meanwhile, patients who required BVS had a higher prevalence of postcardiotomy shock, previous sternotomy, malignancy, and Interagency Registry for Mechanically Assisted Circulatory Support status 1.
Patients managed with BVS had higher central venous pressure and lower tricuspid annulus plane systolic excursion than patients managed with LVS, as well as lower serum albumin and hemoglobin.
Less invasive surgical approaches were used in 71 (33.3%) patients who received LVS and in 10 (6.9%) patients managed with BVS (P <.001). Peripheral arterial outflow cannulation was also more frequent in the LVS group (n=55, 25.8%) than in the BVS group (n=11, 7.6%; P <.001).
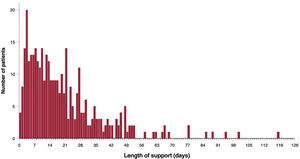
Outcomes of mechanical circulatory supportThe median duration of support was 15 [0-118] days, with no significant differences according to the type of support (LVS group=15 days vs BVS group=15 days; P=.926). Sixty-six (18.6%) patients were assisted for> 30 days and 5 of them underwent elective replacement of a functioning pump. Figure 2 shows the distribution of the duration of support in the study population.
During the in-hospital period after device implantation, 303 (84.6%) patients underwent HTx and 53 (14.8%) died without receiving a transplant. CentriMag LVS was upgraded to CentriMag BVS in 4 patients; of them, only 1 received a transplant, while 3 died during support.
CentriMag was explanted before a donor was found in 5 (1.4%) patients, among whom 1 died shortly after device removal and 2 transitioned to other types of mechanical circulatory support: 1 patient to the HVAD syste, (Medtronic, Ireland) and another to Cardiohelp (Maquet, Germany) venoarterial ECMO, but both died during the index hospital admission. Only 2 (0.6%) patients were discharged alive from hospital after CentriMag explantation, and both died during the following year.
The cumulative rate of HTx during the index hospitalization was 83.1% in the LVS group and 86.9% in the BVS group (P=.328), while the cumulative rate of in-hospital death without HTx was 16% in the LVS group and 13.1% in the BVS group (P=.455). No statistically significant difference was observed in the rate of in-hospital death without HTx between patients who underwent device implantation through full median sternotomy vs less invasive approaches (14.1% vs 17.3%; P=.475).
By means of multivariable logistic regression, we estimated an adjusted odds ratio for in-hospital death without HTx in patients with BVS vs LVS of 0.94 (95%CI, 0.50-1.78).
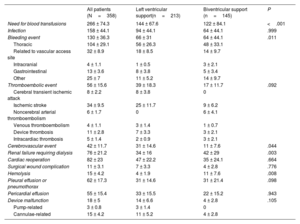
Adverse clinical events associated with mechanical circulatory supportTable 2 shows the cumulative incidence of major adverse clinical events associated with mechanical circulatory support. Patients with BVS had significantly higher rates of bleeding (LVS: 31% vs BVS: 44.1%; P=.011), need for blood transfusions (LVS: 67.4% vs BVS: 84.1%; P <.001), hemolysis (LVS: 1.9% vs BVS: 7.6%; P=.008) and renal failure requiring dialysis (LVS: 16% vs BVS: 29%; P=.003) than patients managed with LVS. The incidence of cerebrovascular events was significantly higher in the LVS group (LVS: 14.6% vs BVS: 7.6%; P=.044).
Cumulative incidence of adverse clinical events associated with mechanical circulatory support in the study population according to the mode of support
| All patients (N=358) | Left ventricular support(n=213) | Biventricular support (n=145) | P | |
|---|---|---|---|---|
| Need for blood transfusions | 266 ± 74.3 | 144 ± 67.6 | 122 ± 84.1 | <.001 |
| Infection | 158 ± 44.1 | 94 ± 44.1 | 64 ± 44.1 | .999 |
| Bleeding event | 130 ± 36.3 | 66 ± 31 | 64 ± 44.1 | .011 |
| Thoracic | 104 ± 29.1 | 56 ± 26.3 | 48 ± 33.1 | |
| Related to vascular access site | 32 ± 8.9 | 18 ± 8.5 | 14 ± 9.7 | |
| Intracranial | 4 ± 1.1 | 1 ± 0.5 | 3 ± 2.1 | |
| Gastrointestinal | 13 ± 3.6 | 8 ± 3.8 | 5 ± 3.4 | |
| Other | 25 ± 7 | 11 ± 5.2 | 14 ± 9.7 | |
| Thromboembolic event | 56 ± 15.6 | 39 ± 18.3 | 17 ± 11.7 | .092 |
| Cerebral transient ischemic attack | 8 ± 2.2 | 8 ± 3.8 | 0 | |
| Ischemic stroke | 34 ± 9.5 | 25 ± 11.7 | 9 ± 6.2 | |
| Noncerebral arterial thromboembolism | 6 ± 1.7 | 0 | 6 ± 4.1 | |
| Venous thromboembolism | 4 ± 1.1 | 3 ± 1.4 | 1 ± 0.7 | |
| Device thrombosis | 11 ± 2.8 | 7 ± 3.3 | 3 ± 2.1 | |
| Intracardiac thrombosis | 5 ± 1.4 | 2 ± 0.9 | 3 ± 2.1 | |
| Cerebrovascular event | 42 ± 11.7 | 31 ± 14.6 | 11 ± 7.6 | .044 |
| Renal failure requiring dialysis | 76 ± 21.2 | 34 ± 16 | 42 ± 29 | .003 |
| Cardiac reoperation | 82 ± 23 | 47 ± 22.2 | 35 ± 24.1 | .664 |
| Surgical wound complication | 11 ± 3.1 | 7 ± 3.3 | 4 ± 2.8 | .776 |
| Hemolysis | 15 ± 4.2 | 4 ± 1.9 | 11 ± 7.6 | .008 |
| Pleural effusion or pneumothorax | 62 ± 17.3 | 31 ± 14.6 | 31 ± 21.4 | .098 |
| Pericardial effusion | 55 ± 15.4 | 33 ± 15.5 | 22 ± 15.2 | .943 |
| Device malfunction | 18 ± 5 | 14 ± 6.6 | 4 ± 2.8 | .105 |
| Pump-related | 3 ± 0.8 | 3 ± 1.4 | 0 | |
| Cannulae-related | 15 ± 4.2 | 11 ± 5.2 | 4 ± 2.8 |
The data are presented as No. (%).
Patients who underwent less invasive device implantation had lower rates of bleeding events (full sternotomy: 39.7% vs less invasive: 24.7%; P=.013), need for transfusions (full sternotomy: 83.4% vs less invasive: 43.2%; P <.001) and cardiac reoperation (full sternotomy: 26.4% vs less invasive: 11.1%; P=.004) than patients who underwent full median sternotomy ().
Device malfunctionEighteen (5%) patients experienced device malfunction. Three cases were due to electrical/mechanical failure of the pump, while the remaining 15 were related to the cannulae: 9 cases of thrombosis, 3 cases of displacement and 3 cases of insufficient blood drainage.
No statistically significant differences were observed in the incidence of device malfunction between patients managed with BVS or LVS (2.8% vs 6.6%; P=.105). However, patients who underwent less invasive implantation had a higher cumulative rate of device malfunction than patients implanted through full median sternotomy (9.9% vs 3.6%; P=.023) figure 3.
Overall, 8 (44.4%) out of the 18 patients who experienced device malfunction died without receiving a transplant. Specific details of episodes of device malfunction are shown in .
Posttransplant survivalOverall, 303 patients underwent emergency HTx during the index hospital admission. Upon HTx surgery, 176 (58.1%) patients were supported with CentriMag LVS and 127 (41.9%) with CentriMag BVS. The mean±standard deviation age of donors was 43.5±12.3 years and 57 (18.8%) were women. Mean ischemic time was 209.4±61.2minutes. No statistically significant differences between BVS and LVS patients were observed regarding donor characteristics (table 1).
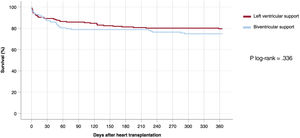
During the first posttransplant year, 68 (22.4%) patients died, 36 (20.5%) in the LVS group and 32 (25.2%) in the BVS group. One-year posttransplant survival curves are represented in figure 4.
No statistically significant differences in 1-year posttransplant survival were observed between candidates bridged with LVS or BVS, according to the log-rank test (P=.336). There were no statistically significant differences in 1-year posttransplant survival between patients who underwent device implantation through median sternotomy vs less invasive surgical approaches (78.8% vs 73.1%; P=.325) or between patients supported with the device for> 30 days vs ≤30 days (75.4% vs 78%; P=.670).
By means multivariable Cox́s regression, we estimated an adjusted hazard ratio for 1-year posttransplant mortality in patients with BVS vs LVS of 1.16 (95%CI, 0.69-1.94).
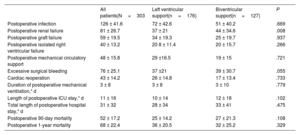
Other postoperative outcomes following transplantationTable 3 shows the incidence of major adverse clinical events during the in-hospital postoperative period after HTx. Patients bridged with BVS showed a statistically significant higher incidence of postoperative renal failure than patients bridged with LVS (34.6% vs 21%; P=.008). No significant differences between the 2 groups were observed regarding the incidence of other in-hospital posttransplant outcomes.
Postoperative outcomes during the in-hospital period following heart transplantation according to the mode of support
| All patients(N=303 | Left ventricular support(n=176) | Biventricular support(n=127) | P | |
|---|---|---|---|---|
| Postoperative infection | 126 ± 41.6 | 72 ± 42.6 | 51 ± 40.2 | .669 |
| Postoperative renal failure | 81 ± 26.7 | 37 ± 21 | 44 ± 34.6 | .008 |
| Postoperative graft failure | 59 ± 19.5 | 34 ± 19.3 | 25 ± 19.7 | .937 |
| Postoperative isolated right ventricular failure | 40 ± 13.2 | 20 8 ± 11.4 | 20 ± 15.7 | .266 |
| Postoperative mechanical circulatory support | 48 ± 15.8 | 29 ±16.5 | 19 ± 15 | .721 |
| Excessive surgical bleeding | 76 ± 25.1 | 37 ±21 | 39 ± 30.7 | .055 |
| Cardiac reoperation | 43 ± 14.2 | 26 ± 14.8 | 17 ± 13.4 | .733 |
| Duration of postoperative mechanical ventilation,* d | 3 ± 8 | 3 ± 8 | 3 ± 10 | .779 |
| Length of postoperative ICU stay,* d | 11 ± 16 | 10 ± 14 | 12 ± 18 | .102 |
| Total length of postoperative hospital stay,* d | 31 ± 32 | 28 ± 34 | 33 ± 41 | .475 |
| Postoperative 90-day mortality | 52 ± 17.2 | 25 ± 14.2 | 27 ± 21.3 | .108 |
| Postoperative 1-year mortality | 68 ± 22.4 | 36 ± 20.5 | 32 ± 25.2 | .329 |
ICU, intensive care unit.
The data are expressed as No. (%) or median [interquartile range].
There were no statistically significant differences in the incidence of adverse postoperative outcomes following HTx between patients who underwent conventional vs less invasive device implantation ().
DISCUSSIONTo the best of our knowledge, this is the largest study focused on the use of the CentriMag acute circulatory support system with the primary intention of bridging to HTx. Our results support the feasibility of this strategy, which showed fairly satisfactory clinical outcomes independently of the mode of support (LVS or BVS) or the type of surgical approach used for device implantation.
Several series of patients treated with the CentriMag system have been published since Mueller et al.13 reported their initial experience 2 decades ago. Until now, the largest single-center experience was reported by the Columbia group.14,15 In a bridge-to-decision setting, these authors observed a frequency of myocardial recovery of ∼30 and a frequency of bridging to heart replacement therapy (HTx or LVAD implantation) of ∼35, as well as an overall 1-year survival rate of ∼50. In a systematic review of 53 observational studies that included 999 patients treated with CentriMag, Borisenko et al.7 reported an overall on-support survival ranging from 62 to 83 depending on the underlying heart condition, with the worst outcomes corresponding to the subgroups of patients with postcardiotomy shock or posttransplant primary graft failure.
In our cohort, on-support survival after CentriMag implantation reached ∼85, a global outcome that seems superior to previous experiences.7,14–17 However, this result is influenced by selection bias, given that patients included in our study were considered suitable candidates for HTx and, indeed, all were waitlisted with high priority. To date, a small study by Def et al.18 has beenthe only one to evaluate the results of the CentriMag system in a specific bridge-to-transplant setting. These authors reported a notable 1-year survival rate of 89 in a single-center cohort of 27 consecutive HTx candidates bridged with the device. However, no specific details on the distribution of pre- and posttransplant deaths were described in this earlier report.
In a context of short waiting list times, as is the case of Spain,4 the use of the CentriMag system is an attractive strategy for bridging acutely compromised candidates to emergency HTx. This temporary device is widely available and easy to implant and provides full circulatory support that can be maintained safely for longer periods than that provided by percutaneous VADs or ECMO, favoring end-organ function recovery and physical rehabilitation. The 30-day officially licensed period for CentriMag use is often sufficiently long to allow an organ to become available; nevertheless, nearly 20 patients in our cohort required support for longer periods, showing comparable outcomes. Other authors have reported good clinical experiences with the use of the CentriMag system beyond 30 days of support.6
The CentriMag system is conceptually intended to provide circulatory support to critically ill patients with cardiogenic shock. However, in our cohort the device was implanted in a few transplant candidates with less severe clinical status, as is the case of those with Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles 3 or 4. At first glance, this could be counterintuitive, as it could be expected that, when deteriorating, these patients should have undergone durable VAD implantation as the first option. This decision was probably influenced by the good performance of the Spanish organ donor retrieval system, which makes it highly likely that status 0 candidates will be allocated a heart graft for transplantation within less than 2 weeks. Of note, the number of durable VADs implanted as a bridge to HTx decreased abruptly in United States after 2018, when a new organ donor allocation protocol that prioritizes HTx candidates on temporary mechanical circulatory support was adopted.19
In our study, 1-year posttransplant survival was ∼78. This result is slightly lower than that of patients undergoing HTx without mechanical circulatory support in Spain in the current era,3 but should be placed in the context of the critically ill condition of emergency HTx candidates, as well as the frequent use of suboptimal donors of advanced age and long cold ischemic times. Indeed, outcomes of patients bridged with CentriMag seemed to be similar to those of other emergency HTx candidates bridged with percutaneous VADs like Impella (Abiomed, United States) and somewhat better than those bridged with venoarterial ECMO.4 The question of whether prioritizing HTx candidates supported with temporary devices is a reasonable strategy or not remains a matter of debate. We believe that the strategy is, indeed, reasonable. Although posttransplant mortality may be increased in these patients, they are probably the subgroup with the most favorable risk-benefit ratio of HTx, given their high expected mortality if they do not receive a transplant.20
Adverse clinical events, whether related to the device itself or to the critically ill condition of the patient, are the Achilles’ heel of mechanical circulatory support. The most frequently reported complications are thrombotic, hemorrhagic, and infectious complications,15 and they also occurred frequently in our cohort. Rates of ischemic stroke, bleeding events and infection were ∼10, ∼36, and ∼44, in agreement with published literature.6,15 Device malfunction leading to a failure of support was rare, and, in most cases, was related to thrombosis or displacement of the cannulae. The incidence of pump failure was very low (< 1), supporting the safety of the system.
The rate of ischemic stroke was higher among patients treated with LVS than among those treated with BVS. This result was influenced by more frequent use of LVS in patients with coronary heart disease and recent myocardial infarction, who, theoretically, are more prone to cerebrovascular events. Ischemic stroke may be the result of arterial manipulation or cannulation, thrombus formation within the pump or along the cannula, or the presence of intracardiac thrombus. Whether the higher use of peripheral cannulation—mainly axillary or subclavian—in patients bridged with LVS might contribute to this phenomenon is uncertain, as it was not clearly associated with an excess of thromboembolic complications in other studies.8
On the other hand, patients requiring BVS showed higher cumulative rates of hemolysis than those treated with LVS, probably in relation to increased shear stress and destruction of blood cells inherent to the use of more complex circulatory systems, which usually require 2 pumps and 3 or 4 vascular cannulae. The high risk of renal failure observed in patients requiring BVS may sometimes be a consequence of hemolysis; however, it is probably more often explainable by the deleterious effect of right ventricular failure on kidney function.21
Bleeding events associated with MCS usually occur in the first few days after device implantation15 and are mainly related to surgical tissue aggression and intrinsic coagulopathy, which is frequent in patients with advanced heart failure and may be aggravated by cardiopulmonary bypass and the use of antithrombotic drugs. In our series, the rate of bleeding events was higher in patients requiring BVS than in those managed with LVS; this finding was probably related to the higher surgical invasivity in the first group,8 also influenced by more compromised liver function as a result of systemic congestion.
In our cohort, less invasive surgical techniques were used mainly for the implantation of LVS circuits and were associated with lower cumulative rates of surgical bleeding, blood transfusions, and cardiac reoperation. However, the potential benefit of these novel approaches in reducing preoperative blood loss may be counterbalanced by an increased risk of device malfunction leading to a failure of support, which in our cohort was mostly caused by problems with the cannulae, such as thrombosis, accidental displacement, or insufficient blood drainage. Indeed, less invasive CentriMag implantation did not have a significant impact on pre- or posttransplant survival in our study. These results must be interpreted carefully, given that the number of less invasive procedures was relatively low in our cohort and that the study period probably involved the learning curve of the surgical technique in most of the participating centers; both circumstances might have led to a higher than expected number of adverse clinical events among patients who underwent less invasive device implantation.
LimitationsThe present investigation has some limitations. First, our analysis was based on a general registry of emergency HTx patients, which was not intentionally designed for the specific purpose of the study. Second, our results may be influenced by the selection, observation, information, and confusion biases inherent to observational studies. Third, the registry was not externally monitored, and the allocation of adverse clinical outcomes was left to the discretion of local investigators. Fourth, we analyzed a heterogeneous population of HTx candidates treated in several different Spanish institutions, with variable local protocols and clinical expertise, and over a long period of inclusion and therefore, outcomes could be affected by significant heterogenicity. Of note, we focused specifically on a bridge-to-transplant setting and all patients included in this study were waitlisted with high priority while on temporary mechanical circulatory support. Finally, given the peculiarities of our national organ sharing network, which is characterized by very short waiting times for prioritized HTx candidates, we cannot be sure that our results are reproducible in other circumstances.
CONCLUSIONSThe present study supports the feasibility of the use of the CentriMag device for bridging patients to high-priority HTx in a setting of short waiting list times. On-device and posttransplant outcomes of this strategy were acceptable, independently of whether the patient required isolated LVS or BVS.
- -
Emergency heart transplantation is an option to be considered in critically ill patients with advanced heart failure who cannot be weaned from temporary mechanical circulatory support.
- -
CentriMag is a continuous-flow, extracorporeal, surgically implanted device that is intended to provide up to 30 days of left ventricular, right ventricular, or biventricular support in patients with cardiogenic shock.
- -
In Spain, the use of CentriMag as a direct bridge to heart transplantation is fairly common. However, there is a lack of information on the results of this therapeutic strategy.
- -
To the best of our knowledge, this is the largest multi-institutional study that reports the results of the use of Centrimag as a direct bridge to heart transplantation to date.
- -
Our study supports the feasibility of this strategy, which was associated with acceptable clinical outcomes, independently of whether the device was used for left ventricular or biventricular support.
The ASIS-TC registry was funded by the Fundación Mutua Madrileña (Madrid, Spain) through 2 competitive research grants (Ayudas para Investigación en Salud, X and XIV annual announcements, years 2014 and 2018), awarded to E. Barge-Caballero, senior author of this manuscript.
AUTHORS’ CONTRIBUTIONSG. Cabezon-Villalba and E. Barge-Caballero contributed equally to the present manuscript.
G. Cabezon-Villalba: conceptualization, manuscript drafting, data collection. E. Barge-Caballero: conceptualization, manuscript drafting, data collection, funding acquisition, statistical analysis, coordination, supervision. L. de la Fuente-Galan: conceptualization, data collection, manuscript editing. M.G. Crespo-Leiro: funding acquisition, data collection, manuscript editing, coordination, supervision. J. Muñiz: funding acquisition, statistical analysis, manuscript editing. All other authors: data collection, manuscript editing.
CONFLICT OF INTERESTSE. Barge-Caballero received an academic grant from Abbot Vascular for taking the Postgraduate Course in Heart Failure 2016-2017 of the University of Zurich, as well as travel grants, consultant fees and speaker fees from Abbot Vascular. J. González-Costello received travel grants, consultant fees and speaker fees from Abbot Vascular. M.G. Crespo-Leiro received travel grants from Abbot Vascular. M.D. García-Cosío Carmena received speaker fees from Abbot Vascular and a travel grant from Epycardio. S. Mirabet-Pérez received travel grants from Abbot Vascular. M.Á. Castel-Lavilla received speaker fees from Microport CRM, Cuquerella Medical Communication and AstraZeneca and travel grants from Abbot Vascular, Epycardio, Novartis, and AstraZeneca.
The authors of this manuscript guarantee that the following investigators are responsible for the data published in this study:
Eduardo Barge-Caballero (Complejo Hospitalario Universitario de A Coruña); José Luis Lambert-Rodríguez (Hospital Universitario Central de Asturias); María Ángeles Castel-Lavilla (Hospital Clinic de Barcelona);José González-Costello (Hospital Universitario de Bellvitge); Francisco González-Vilchez (Hospital Universitario Marqués de Valdecilla); Luis de la Fuente-Galán (Hospital Clínico Universitario de Valladolid); Juan Delgado-Jiménez (Hospital Universitario Doce de Octubre); Luis Almenar-Bonet (Hospital Universitario y Politécnico La Fe); Iris Garrido-Bravo (Hospital Universitario Virgen de la Arrixaca); Sonia Mirabet-Pérez (Hospital Universitario Santa Creu i Sant Pau); Teresa Blasco-Peiró (Hospital Universitario Miguel Servet); Gregorio Rábago Juan-Aracil (Clínica Universidad de Navarra); Diego Rangel-Sousa (Hospital Universitario Virgen del Rocío); Daniela Hervás-Sotomayor (Hospital Universitario Reina Sofía); Manuel Martínez-Sellés (Hospital Universitario Gregorio Marañón); Manuel Gómez-Bueno (Hospital Universitario Puerta de Hierro).
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.05.002