Few data are available on the usefulness of drugs typically used to treat heart failure (HF) in patients with transthyretin cardiac amyloidosis (ATTR-CA). Of these medications, the most pertinent drug class is possibly sodium-glucose cotransporter type 2 inhibitors (SGLT2i) because they are indicated for both heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF).1 The latter is the form with the most frequent clinical presentation in ATTR-CA patients.2 The proven diagnostic benefit of this class of medications in all ejection fraction types, as well as its diuretic effect and favorable hemodynamic profile, suggests that it could be a good therapeutic option in patients with ATTR-CA. However, because this population is systematically excluded from clinical trials of SGLT2i, its effectiveness and safety profile in ATTR-CA are unknown. Accordingly, we examined the prevalence of SGLT2i use and the safety of the drugs in a cohort of patients with ATTR-CA.
We established a prospective registry of all patients diagnosed with ATTR-CA in our center between January 1, 2018, and July 31, 2022, and identified all patients who received any treatment with an SGLT2i. All adverse events potentially associated with this drug class were recorded via a retrospective review of electronic medical records from the date of treatment initiation to discontinuation, patient death, or end of the observation period, established at November 30, 2022. Participant follow-up was conducted in the Heart Failure Unit at least every six months. All patients signed an informed consent form before their inclusion in the registry.
Of a total of 176 patients with ATTR-CA, 64 (36.4%) had received a treatment with an SGLT2i (47 [73.4%] with empagliflozin, 16 [25%] with dapagliflozin, and 1 [1.6%] with canagliflozin). Of these, 13 were already receiving the drug at the time of their inclusion in the registry and 51 were prescribed it later. The median time to drug initiation was 940 [interquartile range, 377-1176] days. The most common indication was HFpEF (28 patients, 43.8%), followed by HFrEF (19 patients, 29.7%) and diabetes mellitus type 2 (17 patients, 26.6%).
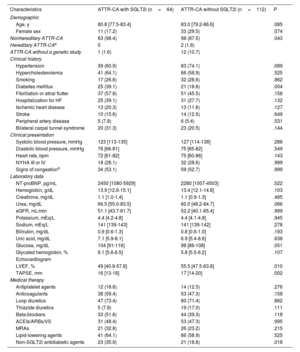
The median patient age was 80.8 years and there were 11 women (17.2%). At the time of registry inclusion, most patients (46 [71.9%]) were in New York Heart Association class I or II, 34 (53.1%) had signs or symptoms of congestion, and 25 (39.1%) had already been hospitalized at least once for HF. The most frequently prescribed drug class was loop diuretics (47 patients, 73.4%), while neurohormonal modulators were used in less than half of the cohort. The baseline characteristics of the population are shown in table 1.
Patients’ baseline characteristics at the time of registry inclusion
| Characteristics | ATTR-CA with SGLT2i (n=64) | ATTR-CA without SGLT2i (n=112) | P |
|---|---|---|---|
| Demographic | |||
| Age, y | 80.8 [77.5-83.4] | 83.0 [79.2-86.6] | .085 |
| Female sex | 11 (17.2) | 33 (29.5) | .074 |
| Nonhereditary ATTR-CA | 63 (98.4) | 98 (87.5) | .043 |
| Hereditary ATTR-CAa | 0 | 2 (1.8) | |
| ATTR-CA without a genetic study | 1 (1.6) | 12 (10.7) | |
| Clinical history | |||
| Hypertension | 39 (60.9) | 83 (74.1) | .089 |
| Hypercholesterolemia | 41 (64.1) | 66 (58.9) | .525 |
| Smoking | 17 (26.6) | 32 (28.6) | .862 |
| Diabetes mellitus | 25 (39.1) | 21 (18.8) | .004 |
| Fibrillation or atrial flutter | 37 (57.8) | 51 (45.5) | .158 |
| Hospitalization for HF | 25 (39.1) | 31 (27.7) | .132 |
| Ischemic heart disease | 13 (20.3) | 13 (11.6) | .127 |
| Stroke | 10 (15.6) | 14 (12.5) | .649 |
| Peripheral artery disease | 5 (7.8) | 6 (5.4) | .531 |
| Bilateral carpal tunnel syndrome | 20 (31.3) | 23 (20.5) | .144 |
| Clinical presentation | |||
| Systolic blood pressure, mmHg | 123 [113-135] | 127 [114-138] | .286 |
| Diastolic blood pressure, mmHg | 76 [66-81] | 75 [65-82] | .549 |
| Heart rate, bpm | 72 [61-82] | 75 [60-86] | .143 |
| NYHA III or IV | 18 (28.1) | 32 (28.6) | .999 |
| Signs of congestionb | 34 (53.1) | 59 (52.7) | .999 |
| Laboratory data | |||
| NT-proBNP, pg/mL | 2450 [1080-5929] | 2280 [1007-4503] | .522 |
| Hemoglobin, g/dL | 13.9 [12.6-15.1] | 13.4 [12.1-14.8] | .103 |
| Creatinine, mg/dL | 1.1 [1.0-1.4] | 1.1 [0.9-1.3] | .485 |
| Urea, mg/dL | 66.5 [55.0-83.5] | 60.0 [48.2-84.7] | .066 |
| eGFR, mL/min | 51.1 [43.7-61.7] | 52.2 [40.1-65.4] | .999 |
| Potassium, mEq/L | 4.4 [4.2-4.8] | 4.4 [4.1-4.8] | .945 |
| Sodium, mEq/L | 141 [139-143] | 141 [139-142] | .278 |
| Bilirubin, mg/dL | 0.9 [0.6-1.3] | 0.8 [0.6-1.0] | .193 |
| Uric acid, mg/dL | 7.1 [5.9-8.1] | 6.9 [5.4-8.6] | .638 |
| Glucose, mg/dL | 104 [91-116] | 98 [88-108] | .051 |
| Glycated hemoglobin, % | 6.1 [5.6-6.5] | 5.8 [5.5-6.2] | .107 |
| Echocardiogram | |||
| LVEF, % | 49 [40.9-57.8] | 55.5 [47.5-63.8] | .010 |
| TAPSE, mm | 16 [13-18] | 17 [14-20] | .002 |
| Medical therapy | |||
| Antiplatelet agents | 12 (18.8) | 14 (12.5) | .276 |
| Anticoagulants | 38 (59.4) | 53 (47.3) | .158 |
| Loop diuretics | 47 (73.4) | 80 (71.4) | .862 |
| Thiazide diuretics | 5 (7.8) | 19 (17.0) | .111 |
| Beta-blockers | 33 (51.6) | 44 (39.3) | .118 |
| ACEIs/ARBs/VS | 31 (48.4) | 53 (47.3) | .995 |
| MRAs | 21 (32.8) | 26 (23.2) | .215 |
| Lipid-lowering agents | 41 (64.1) | 66 (58.9) | .525 |
| Non-SGLT2i antidiabetic agents | 23 (35.9) | 21 (18.8) | .018 |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; ATTR-CA, transthyretin cardiac amyloidosis; eGFR, estimated glomerular filtration rate by the Cockcroft-Gault equation; HF, heart failure; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SGLT2i, sodium-glucose cotransporter-2 inhibitor; TAPSE, tricuspid annular plane systolic excursion; VS, valsartan/sacubitril.
Categorical variables are presented as No. (%) and continuous variables as median [interquartile range].
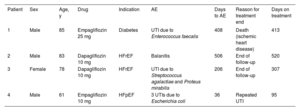
During a median SGLT2i treatment time of 304 [76-482] days, 3 patients (4.7%) had a urinary infection and 1 (1.6%) had a genital infection (table 2). This drug class was not associated with fracture, ketoacidosis, hypoglycemia, hypotension, amputation, or Fournier gangrene. In addition, 2 patients (3.1%) discontinued the SGLT2i therapy, 1 due to repeat urinary infections (Case No. 4 of table 2) and the other (a 64-year-old man taking empagliflozin 10mg for HFpEF) of his own accord and without clinical justification.
Detailed description of the adverse events observed in the cohort
| Patient | Sex | Age, y | Drug | Indication | AE | Days to AE | Reason for treatment end | Days on treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 85 | Empagliflozin 25 mg | Diabetes | UTI due to Enterococcus faecalis | 408 | Death (ischemic heart disease) | 413 |
| 2 | Male | 83 | Dapagliflozin 10 mg | HFrEF | Balanitis | 506 | End of follow-up | 520 |
| 3 | Female | 78 | Dapagliflozin 10 mg | HFrEF | UTI due to Streptococcus agalactiae and Proteus mirabilis | 206 | End of follow-up | 307 |
| 4 | Male | 61 | Empagliflozin 10 mg | HFpEF | 3 UTIs due to Escherichia coli | 36 | Repeated UTI | 95 |
AE, adverse event; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; UTI, urinary tract infection.
The median follow-up of the cohort from inclusion in the registry was 790 [401-1245] days and 45 deaths occurred in total (25.6%) (6 in the SGLT2i group [9.4%] and 39 in the non-SGLT2i group [34.8%]).
In summary, our single-center and prospective cohort of patients with ATTR-CA revealed that slightly more than a third of participants were treated with an SGLT2i and that these drugs are safe and well-tolerated. The only adverse events observed were urinary and genital infections and only 1 patient discontinued the treatment. A review of the literature revealed just 2 studies that also included patients with ATTR-CA treated with SGLT2is. In the first,3 15 patients with this heart condition and diabetes mellitus received a member of this drug class (dapagliflozin in 53%) during a median of 8 months. No urinary or genital infections were detected and the treatment was discontinued in 2 patients due to the development of constipation and HF deterioration, respectively. In the second study,4 clinical course and biomarkers were compared between 17 patients with ATTR-CA treated with dapagliflozin and 40 patients with ATTR-CA who were not treated with dapagliflozin. The authors did not detect any adverse events associated with the drug during a median treatment duration of 3 months.
The main limitations of the current study are the small sample size and its single-center design, which limits the extrapolation of the results to other populations. The main strength of the study is that, to date, it includes the largest series of patients with ATTR-CA treated with an SGLT2i and that the results indicate that these drugs are safe and well-tolerated in this population. Observational studies with a larger sample size and longer follow-up duration are required, as well as clinical trials, to verify our hypothesis and assess the effects of these drugs on cardiovascular morbidity and mortality.
FUNDINGThe authors did not receive specific funding for the preparation of this manuscript.
ETHICAL CONSIDERATIONSThe authors of this article accept full responsibility for its content as defined by the International Committee of Medical Journal Editors. The included patients signed an informed consent form before their participation. Possible sex and gender biases have been considered.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence was not used.
AUTHORS’ CONTRIBUTIONSAll authors have contributed equally to the drafting of this document.
CONFLICTS OF INTERESTThe authors do not declare conflicts of interests in relation to this work.