Prasugrel and ticagrelor are the drugs of choice for acute coronary syndrome, but they have a more limited profile than clopidogrel due to the risk of bleeding.1 The percentage of patients whose clinical characteristics could limit or contraindicate the use of the new antiplatelet agents is unknown. We analyzed this percentage in a unselected cohort of consecutive patients from several Spanish centers with different forms of acute coronary syndrome.
From October 1, 2013, we studied 25 consecutive patients diagnosed with any form of acute coronary syndrome in 17 hospitals with a cardiac catheterization laboratory, 1 in each autonomous region. The only patients excluded were those taking oral anticoagulants. We studied their baseline characteristics, antiplatelet therapy, and the characteristics that could limit or contraindicate use of the new antiplatelet agents.
Prasugrel was considered as a nonindication, based on its product information sheet, as was not performing percutaneous coronary intervention, whereas active bleeding and a history of stroke and transient ischemic attack (TIA) were considered as contraindications. Age ≥ 75 years and weight < 60kg were considered to be limitations. According to the product information sheet, ticagrelor is contraindicated in active pathological bleeding and previous intracranial hemorrhage. On the basis of data provided by the literature, a history of TIA or nonbleeding stroke2,3 was considered to be a limitation, as well as moderate or severe bronchopathy4 and glomerular filtration rate ≤ 30mL/h.5
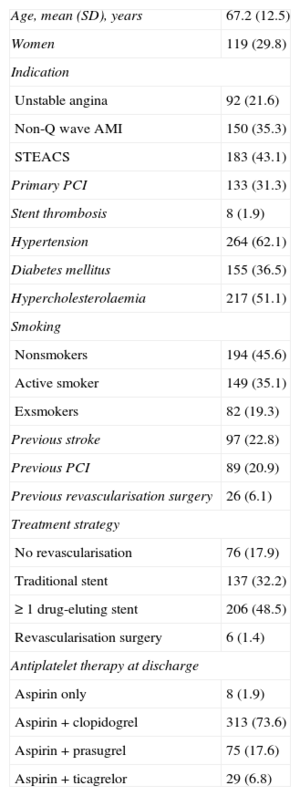
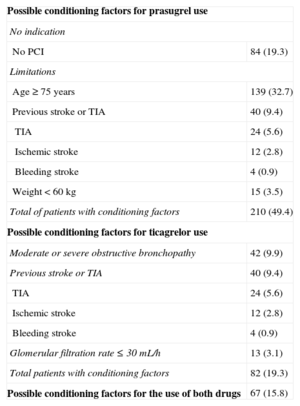
We studied 425 patients. The baseline characteristics, treatment strategy, and antiplatelet therapy are shown in Table 1 and the conditioning factors are shown in Table 2. A total of 210 patients (49.4%) were deemed ineligible for prasugrel, 84 (19.3%) for not having undergone percutaneous coronary intervention, 139 (32.7%) for being ≥ 75 years, 15 (3.5%) for weighing < 60kg, and 40 (9.4%) for having a history of TIA or stroke. With ticagrelor, of 82 patients (19.3%), 42 (9.9%) could have limitations due to moderate or severe obstructive pulmonary disease, 40 (9.4%) due to stroke or TIA, and 13 (3.1%) due to glomerular filtration rate < 30mL/h. There was wide variability between centers in the use of these drugs (from 5%-60%).
Clinical Characteristics and Antiplatelet Agents (n = 425)
| Age, mean (SD), years | 67.2 (12.5) |
| Women | 119 (29.8) |
| Indication | |
| Unstable angina | 92 (21.6) |
| Non-Q wave AMI | 150 (35.3) |
| STEACS | 183 (43.1) |
| Primary PCI | 133 (31.3) |
| Stent thrombosis | 8 (1.9) |
| Hypertension | 264 (62.1) |
| Diabetes mellitus | 155 (36.5) |
| Hypercholesterolaemia | 217 (51.1) |
| Smoking | |
| Nonsmokers | 194 (45.6) |
| Active smoker | 149 (35.1) |
| Exsmokers | 82 (19.3) |
| Previous stroke | 97 (22.8) |
| Previous PCI | 89 (20.9) |
| Previous revascularisation surgery | 26 (6.1) |
| Treatment strategy | |
| No revascularisation | 76 (17.9) |
| Traditional stent | 137 (32.2) |
| ≥ 1 drug-eluting stent | 206 (48.5) |
| Revascularisation surgery | 6 (1.4) |
| Antiplatelet therapy at discharge | |
| Aspirin only | 8 (1.9) |
| Aspirin + clopidogrel | 313 (73.6) |
| Aspirin + prasugrel | 75 (17.6) |
| Aspirin + ticagrelor | 29 (6.8) |
AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation; STEACS, ST-segment elevation acute coronary syndrome.
Unless otherwise indicated, values express No. (%).
Conditioning Factors for the Use of New Antiplatelet Agents
| Possible conditioning factors for prasugrel use | |
| No indication | |
| No PCI | 84 (19.3) |
| Limitations | |
| Age ≥ 75 years | 139 (32.7) |
| Previous stroke or TIA | 40 (9.4) |
| TIA | 24 (5.6) |
| Ischemic stroke | 12 (2.8) |
| Bleeding stroke | 4 (0.9) |
| Weight < 60 kg | 15 (3.5) |
| Total of patients with conditioning factors | 210 (49.4) |
| Possible conditioning factors for ticagrelor use | |
| Moderate or severe obstructive bronchopathy | 42 (9.9) |
| Previous stroke or TIA | 40 (9.4) |
| TIA | 24 (5.6) |
| Ischemic stroke | 12 (2.8) |
| Bleeding stroke | 4 (0.9) |
| Glomerular filtration rate ≤ 30 mL/h | 13 (3.1) |
| Total patients with conditioning factors | 82 (19.3) |
| Possible conditioning factors for the use of both drugs | 67 (15.8) |
PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
The values express No. (%).
Because TRITON (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel) only included patients with percutaneous interventions and recorded bleeding complications in some subgroups, the profile of prasugrel is more limited than that of clopidogrel. In our series, one third of the patients was aged 75 years or older, almost 1 of 10 had a history of TIA or stroke, and 3.5% weighed < 60kg. Adding an extra 20% due to the absence of percutaneous coronary intervention, these aspects excluded almost half of the patients and underscore the need for attention in prescribing, as demonstrated by the PINNACLE study,6 an American registry of 27 533 patients treated with prasugrel, in which 13.9% had a history of TIA or stroke. These aspects can acquire particular importance in ST-segment elevation myocardial infarction, because it may be difficult to obtain a complete medical history in emergency situations.
Ticagrelor differs from clopidogrel and is similar to adenosine. In addition to its antiplatelet effect, it inhibits adenosine reuptake by red blood cells, which increases plasma concentrations. Because a benefit was demonstrated in the PLATO study (Platelet Inhibition and Patient Outcomes) both with invasive and conventional handling, ticagrelor is indicated in all types of acute coronary syndrome in the absence of contraindications such as active pathological bleeding and prior intracranial bleeding. In our series, 0.9% had a contradiction due to an event of this kind in their medical history.
Based on data in the literature, we also considered the presence of moderate or severe obstructive pulmonary disease, renal failure with glomerular filtration rate < 30mL/h, TIA, and ischemic stroke as possible limitations. In the PLATO study, 14.5% of patients on ticagrelor and 8.7% of those on clopidogrel reported dyspnea, and 0.4% and 0.3% were classified as severe.4 However, most cases of dyspnea are mild and, because they do not cause structural lung changes, are reversible on treatment withdrawal. Secondly, although ticagrelor is more effective in renal failure, only 214 patients with glomerular filtration rate < 30mL/h were included, and the bleeding frequency with ticagrelor (20%) was double that of clopidogrel (10%).5 Finally, it also seems reasonable to consider a possible limitation in cases of previous stroke or TIA, as only 6.2% of patients with a history of these events was included, and, although the incidence of complications was low,3 there were more intracranial bleeds with ticagrelor.2 In fact, in the PEGASUS study, which studied ticagrelor vs clopidogrel in 21 000 patients with a history of infarction, the sponsor excluded patients with a prior stroke. In our study, the possible limitation due to glomerular filtration rate < 30mL/h, prior stroke or TIA, and moderate or severe bronchial disease represented 3.1%, 9.4%, and 9.9%, respectively, representing 19.5% of patients with a potential limitation.
In conclusion, apart from the financial considerations, there are other limiting factors with new antiplatelet agents in acute coronary syndrome, which could affect almost half of the patients taking prasugrel and 1 in 5 patients treated with ticagrelor. The use of the new antiplatelet agents varies greatly among hospitals.
Contributors: Felipe Hernández-Hernández, Pilar Carrillo-Sáez, Ignacio Cruz-González, Eduardo Pinar-Bermúdez, Garikoitz Lasa-Larraya, Pilar Mañas, Valeriano Ruiz-Quevedo, Jeremías Bayón, Sergio Rodríguez-Leiras, Silvia Gopar-Gopar, and Esther Sánchez-Insa.