This article reviews the most relevant publications and studies in the field of interventional cardiology in 2014. In the area of coronary interventional procedures, integrated treatment of acute coronary syndrome continues to be the subject of numerous studies that evaluate different devices and pharmacological and mechanical strategies that can be used without increasing the risk of hemorrhage or the need for reintervention. Certain anatomical substrates continue to generate a considerable number of publications, both on the outcomes with different stents and on the use of specific techniques. Bioabsorbable drug-eluting stents are used in increasingly complex lesions with promising results. The development of interventional procedures for structural heart disease continues to advance, with new evidence on percutaneously placed aortic valve prostheses, the outcome of percutaneous mitral valve repair, and the safety and efficacy of left atrial appendage occlusion. Finally, renal denervation has generated one of the major debates of the year.
Keywords
The ATLANTIC (Administration of Ticagrelor in the cath Lab or in the Ambulance for New ST elevation myocardial Infarction to open the Coronary artery) study, an international, multicenter, randomized trial, included 1862 patients with ST-segment elevation acute myocardial infarction (STEMI) with onset less than 6h earlier, and compared prehospital treatment with ticagrelor (in the ambulance) with in-hospital administration (in the catheterization laboratory).1–5 The primary end points were a 70% or greater resolution of ST-segment elevation prior to percutaneous coronary intervention (PCI) and the proportion of patients who did not have TIMI (Thrombolysis In Myocardial Infarction) grade 3 flow in the culprit artery. The secondary end points were the rates of major cardiovascular adverse events and definite stent thrombosis at 30 days. The median time from randomization to angiography was 48min and the mean difference in time between the 2 treatment strategies was 31min. The comparison between prehospital and in-hospital treatment revealed no significant differences in the primary end points. The incidence of definite stent thrombosis was lower in the prehospital group than in the in-hospital group (0.0% and 0.8%, respectively, in the first 24h and 0.2% and 1.2% at 30 days; P=.02). The rates of major bleeding were low and practically identical in the 2 groups. Prehospital administration of ticagrelor to patients with STEMI appeared to be safe but did not improve pre-PCI coronary reperfusion.
CvLPRIT was a prospective, randomized, multicenter trial involving patients with STEMI.6 The objective was to determine the optimal treatment for lesions unrelated to myocardial infarction detected during primary PCI.2,6 Patients were included when multivessel disease was observed, and were randomized either to treatment of the culprit artery alone (n=146) or to complete revascularization (n=150). The primary end point was the composite of all-cause mortality, recurrent myocardial infarction, heart failure, or the need for revascularization at 12 months. Complete revascularization (in the same procedure in 59% of the patients and during admission in the remainder) was associated with a better primary outcome (10.0% vs 21.3%; P=.009).
The NOMI trial7 evaluated the hypothesis that inhaled nitric oxide could reduce myocardial reperfusion injury in STEMI patients undergoing primary PCI.1,7 The study included 250 patients (arriving<12h after symptom onset) who were randomized to receive oxygen delivered by face mask, with or without nitric oxide (at a concentration of 80 parts per million). Gas administration commenced in the catheterization laboratory prior to PCI and continued for 4hours after reperfusion. Infarction size and left ventricular remodeling were evaluated using cardiac magnetic resonance and, 48hours to 72hours after the procedure, there were no differences between the groups with and without nitric oxide in terms of infarction size (18.0% vs 19.4%; P=.44).
Non—ST-segment Elevation Myocardial InfarctionThe FAMOUS-NSTEMI trial8 compared fractional flow reserve (FFR) with angiography for the guidance of management decisions to optimize prognosis in non—ST-segment elevation myocardial infarction. A total of 350 patients with ≥ 1 coronary stenoses ≥ 30% of the lumen diameter, according to visual assessment carried out in 6 hospitals in the United Kingdom, were randomized 1:1 for inclusion in this prospective study. The FFR was measured and the result was revealed to the operator in the FFR-guided group (n = 176), but not to the operator in the angiography-guided group (n=174). For the primary end point, the proportion of patients who initially received medical treatment was higher in the FFR-guided group than in the angiography-guided group (22.7% vs 13.2%; P=.022). Twelve months later, the rate of revascularization continued to be lower in the FFR-guided group. There were no differences between the 2 groups with respect to clinical course and quality of life.
Vascular AccessThe randomized ISAR-CLOSURE trial9 compared manual femoral hemostasis with 2 devices (1 intravascular and the other extravascular) in more than 4500 patients after coronary angiography with a 6-Fr introducer sheath. The primary end point was the composite of vascular complications of any type occurring during the first 30 days after the procedure. These events were recorded in 6.9% of the patients in the device group vs 7.9% of those in the manual compression group (P=nonsignificant). The compression time was significantly shorter in the group in which either of the 2 devices was used and, when compared with one another, the failure rate was lower with the intravascular device than with the extravascular device (5.3% and 12.2%, respectively; P<.001).
Coronary Artery Disease: Specific LesionsLeft Main Coronary ArteryA meta-analysis, including more than 2300 patients (drawn from randomized trials and clinical registries) who underwent elective PCI with drug-eluting stents (DES) to treat severe distal lesions in unprotected left main coronary artery, evaluated the outcomes with a single stent vs a double stent strategy.10 After a mean follow-up of 32 months, the combined incidence of adverse cardiac events was significantly lower in the single stent strategy (20.4% vs 32.8%; P<.001), as was the need for revascularization (10.1% vs 24.3%; P<.001).
RestenosisThe RIBS IV trial11 compared treatment with a paclitaxel-eluting balloon (SeQuent® Please, B. Braun Surgical) and an everolimus-eluting stent (Xience PrimeTM, Abbott Vascular) in patients with restenosis of a DES. The trial included 309 patients with DES restenosis, randomized to either of the 2 strategies. A 90% of the patients underwent angiographic follow-up. At 9 months, the everolimus-eluting stent group had a greater minimal lumen diameter than the paclitaxel-eluting balloon group (2.03mm and 1.80mm, respectively; P=.004) and, at 1 year, a higher proportion of the DES patients had had no need for reintervention (96% vs 87%; P=.008) or no major cardiac events—a composite of cardiac death, myocardial infarction, and culprit vessel revascularization—(90% vs 82%; P=.044).
Chronic OcclusionsFor the prospective CTO-IVUS study,12 once the guide wire had successfully crossed the occlusion, 402 patients with chronic coronary occlusion were randomized to intravascular ultrasound-guided PCI or to angiography-guided PCI. In addition, the patients were randomized to implantation of zotarolimus- or biolimus-eluting stents. The primary end point, the composite of cardiac death, myocardial infarction, or target vessel revascularization within 12 months, occurred less frequently in the intravascular ultrasound-guided group than in the angiography-guided group (2.6% and 7.1%, respectively; P=.035). The rate of crossover from angiography to intravascular ultrasound was significantly higher than that of intravascular ultrasound to angiography guidance (17.4% and 2.5%, respectively; P<.001). The per protocol analysis revealed a lower incidence of the primary end point in the intravascular ultrasound group than in the angiography group (2.2% and 8.4%, respectively; P=.005).
BifurcationsThe prospective, randomized BABILON trial13 compared the clinical and angiographic outcomes of treatment of bifurcation lesions with a paclitaxel-coated balloon plus bare metal stent vs everolimus-eluting stent implantation. The primary end point was lumen loss in the main branch on angiography at 9 months, with a mean (standard deviation) of 0.31 (0.48) mm in the paclitaxel-coated balloon group and 0.16 (0.38) mm in the DES group (P=.15). There were no significant differences in the side branch. However, the incidence of the composite secondary end point (cardiac death, myocardial infarction, or need for revascularization) was significantly higher in the paclitaxel-coated balloon group due to a higher rate of restenosis (13.5% and 1.8%, respectively; P=.027) and need for revascularization (15.4% and 3.6%; P=.045).
Drug-eluting StentsThe prospective, randomized CENTURY II trial14 compared the noninferiority of the Ultimaster DES (Terumo, Tokyo, Japan) with sirolimus and a bioresorbable polymer, with the Xience DES (Abbott Vascular) with everolimus and a permanent polymer. Over 1100 patients were included; for the primary end point (a composite of cardiac death, myocardial infarction, or target vessel revascularization), the Ultimaster DES was not inferior to the Xience DES (4.36% and 5.27%, respectively; P<.0001) at 9 months. The incidence of the composite of cardiac death and myocardial infarction was 2.9% and 3.8%, respectively (P=.40), and that of the need for revascularization was 4.5% and 4.2% (P=.77). The thrombosis rate was 0.9% in both groups.
The aim of the BIOSCIENCE trial15 was to compare the safety and efficacy of a novel ultrathin, cobalt-chromium DES that releases sirolimus from a biodegradable polymer (Orsiro, Biotronik) with a thin-strut, durable-polymer, everolimus-eluting stent (Xience, Abbott Vascular). The study included 2119 patients with 3139 lesions who were assigned to the sirolimus-eluting stent group (1063 patients, 1594 lesions) or the everolimus-eluting stent group (1056 patients, 1545 lesions). The STEMI was the first symptom in 19% of the patients. The primary end point (defined as target lesion failure) was the composite of cardiac death, target vessel-related myocardial infarction, or target vessel revascularization at 12 months and, in this respect, there were no significant differences between the 2 groups (6.5% and 6.6%; P for noninferiority<.0004). No differences were observed in the rates of definite stent thrombosis (0.9% and 0.4%, respectively; P=.16). In the subgroup of patients with STEMI, the sirolimus-eluting stent was associated with a better outcome (3.3% vs 8.7%; P=.024).
Bioabsorbable StentsThe GHOST-EU registry16 included 1189 patients who had received 1 or more bioabsorbable stents—or bioresorbable vascular scaffolds (Absorb BVS, Abbott Vascular). The primary end point was target lesion failure, defined as the composite of cardiac death, myocardial infarction, or target lesion revascularization. A total of 1731 Absorb devices were implanted (mean pressure, 12.3 [3.4] atmospheres), and success was achieved in 99.7% of the cases. The primary end point was recorded in 4.4% of the patients at 6 months (median 109 days), with an annualized rate of 10.1%. Diabetes mellitus was the only independent predictor of the primary end point (P=.006). The incidence of definite or probable thrombosis reached 2.1% at 6 months, with a median of 6.5 days.
The randomized, multicenter ABSORB II trial17 included 501 patients with 1 or 2 lesions in native coronary arteries, who were assigned in a 2:1 ratio to receive a bioabsorbable (Absorb BVS, Abbott Vascular) or an everolimus-eluting stent (Xience, Abbott Vascular). The acute lumen gain was lower in the Absorb group according to quantitative coronary angiography (1.15mm vs 1.46mm; P<.0001) and intravascular ultrasound (2.85mm2 and 3.6mm2, respectively; P<.0001), and the results were a smaller lumen diameter and lumen area following the procedure. However, at 1 year, the rates of angina were lower in the Absorb group (22% vs 30%; P=.04), although this was a post-hoc finding as it was not preestablished. The incidence of the composite end point with the 2 stents was similar (4.8% vs 3.0%; P=.35), and the 2 groups did not differ significantly in terms of the rate of the overall composite of death, myocardial infarction, or any revascularization) (7.3% and 9.1%, respectively; P=.47).
Percutaneous Interventions in Coronary Artery Disease: Antiplatelet and Anticoagulation TherapyThe ISAR TRIPLE trial18,19 randomized 614 patients who had undergone PCI with DES to receive acetylsalicylic acid and a vitamin K antagonist, plus clopidogrel for 6 weeks (n=307) or for 6 months (n=307). After 9 months of follow-up, there were no significant differences between the 2 groups with respect to the composite primary end point of death, myocardial infarction, stent thrombosis, stroke, or TIMI major bleeding (9.8% vs 8.8%; P=.63). Moreover, no significant differences were observed in the secondary end point of TIMI major bleeding (5.3% and 4%; P=.44).
The SECURITY trial20 evaluated the noninferiority of dual antiplatelet therapy for 6 or 12 months in patients treated with PCI and second-generation DES. The primary end point was a composite of cardiac death, myocardial infarction, stroke, stent thrombosis, or major bleeding at 12 months. A total of 1399 patients were randomized to receive dual platelet therapy for 6 months (n=682) or 12 months (n=717). The primary end point was recorded in 4.5% and 3.7% of the patients, respectively, (P=.47) at 12 months, confirming the noninferiority hypothesis. Moreover, there were no differences in the incidence of stent thrombosis at 12 months (0.3% and 0.4%; P=.69) or 24 months (0.1% and 0.0%; P=.31).
The prospective Spanish ESTROFA DAPT registry included consecutive patients treated with new generation DES who, after hospital discharge, received dual antiplatelet therapy for 6 months. The patient selection criteria were silent ischemia, stable angina, low-risk acute coronary syndrome, or acute coronary syndrome with high risk of bleeding. A propensity analysis was carried out with the patients from the ESTROFA-2 study (4354 patients treated with second-generation DES and at least 12 months of dual antiplatelet therapy), to compare the results with a 12-month strategy.21,22 A well balanced cohort of 2572 matched patients (1286 in each group) was included. The rate of definite stent thrombosis after 12 months was 0.4% in the 6-month group and 0.6% in the 12-month group (P=.4), and the incidence of definite or probable thrombosis was 0.7% and 1.5%, respectively (P=.09).
The BRIGHT trial23 evaluated the hypothesis that bivalirudin is superior to heparin in monotherapy or to heparin plus tirofiban in terms of the composite end point of ischemic and bleeding events in patients who undergo emergency PCI to treat acute coronary syndrome. In all, 2194 patients were randomized to the 3 groups (1:1:1). The majority (89%) presented with STEMI, the transradial approach was employed in nearly 80%, and bivalirudin infusion was continued after PCI (mean, 234min) in the corresponding group. At 30 days, the composite end point (major cardiac and cardiovascular events and bleeding) was detected in significantly fewer cases in the bivalirudin group compared with heparin monotherapy and heparin plus tirofiban (8.8% vs 13.2% vs 17.0%; P=.001). This is mainly due to the reduction in bleeding events in the bivalirudin group since, at 1 year, the rates of major ischemic events were similar in the 3 groups (6.7%, 7.3%, and 6.8%, respectively; P=.9).
The HEAT-PPCI trial24 randomized 1829 STEMI patients who underwent emergency coronary angiography to treatment with bivalirudin or heparin. Primary PCI was performed in 83% and 82% of the patients, respectively. There were no significant differences in the use of glycoprotein IIb/IIIa inhibitors (13% and 15%). The primary efficacy end point (major adverse cardiac events) at 4 weeks occurred in 8.7% of the bivalirudin group vs 5.7% of the heparin group (P=.01), mainly due to the increase in stent thrombosis (3.4 vs 0.9%, P=0.001), most of which were acute. The primary safety end point (major bleeding) occurred in 3.5% and 3.1%, respectively (P=.59).
The DAPT study25 included 9961 patients who had undergone implantation of at least 1 DES and who, after 12 months of treatment with acetylsalicylic acid and a thienopyridine (clopidogrel or prasugrel), were randomly assigned to receive thienopyridine therapy or placebo for 18 more months. All the patients continued to take acetylsalicylic acid. The coprimary end point of the study was stent thrombosis and a composite of events (death, myocardial infarction, or stroke) during the period from month 12 to month 30. The primary safety end point was moderate or severe bleeding. Compared with placebo, continued thienopyridine therapy reduced the incidence of stent thrombosis (0.4% vs 1.4%; P<.001) and of major cardiovascular and cerebrovascular events (4.3% vs 5.9%; P<.001). The incidence of myocardial infarction was also lower with thienopyridine therapy than with placebo (2.1% and 4.1%, respectively; P<.001). The rate of all-cause mortality was 2.0% in the group receiving continued thienopyridine therapy and 1.5% in the placebo group (P=.052), but the rate of cardiac death did not differ (0.9% and 1.0%; P=.98). The incidence of moderate or severe bleeding was higher in the thienopyridine group (2.5% vs 1.6%; P=.001).
Intracoronary Diagnostic TechniquesThe FAME 2 trial26 included 1220 patients with stable coronary artery disease who underwent evaluation of all angiographically visible stenoses with FFR. The hypothesis was that FFR-guided PCI would be superior to medical treatment. Patients who had at least 1 stenosis with an FFR ≤ 0.80 were randomized to FFR-guided PCI plus medical treatment or medical treatment alone. The patients with FFR>0.80 in all the lesions were treated medically and included in a registry. The primary end point was a composite of death, myocardial infarction, or urgent revascularization within 2 years of randomization, and its incidence was significantly lower in the PCI group than in the medical treatment group (8.1% vs 19.5%; P<.001). This finding was due to a reduction in the need for urgent revascularization in the PCI group (4.0% vs 16.3%; P<.001), with no significant differences between the groups with regard to rates of death or myocardial infarction. In a specific analysis, the rate of death or myocardial infarction between 8 days and 2 years was lower in the PCI group than in the medical treatment group (4.6% vs 8.0%; P=.04). Finally, among the patients included in the registry, the rate of the primary end point at 2 years was 9%.
The OCT-STEMI trial27 included 201 patients with STEMI who, following emergency coronary angiography, were randomized to primary PCI with or without optical coherence tomography guidance. Analysis of the tomography data revealed that the patients in this group received a higher number of stents, but that the incidence of major adverse cardiac events was similar in the 2 groups, with a low rate of binary stenosis in both (2% vs 3%; P=nonsignificant) at 9 months.
Structural Cardiac InterventionsTranscatheter Aortic Valve ImplantationThe US CoreValve trial28 compared transcatheter aortic valve implantation (TAVI), using a self-expanding bioprosthesis, with surgical aortic valve replacement in patients with severe aortic stenosis and at high surgical risk (according to the Society of Thoracic Surgeons score).28 After randomization of 795 patients, the primary end point was death from any cause at 1 year, which occurred in significantly fewer patients in the transcatheter group than in the surgical group (14.2% and 19.3%, respectively; P=.04). Although the need for a permanent pacemaker was recorded more frequently in the transcatheter group (22.3% vs 11.3% at 1 year; P<.001), there was a significantly higher incidence of major bleeding, renal failure, and development of atrial fibrillation in the surgical group.
The objective of the CHOICE clinical trial,29 which compared the Edwards Sapien XT valve with the CoreValve system, was to determine whether a balloon-expandable valve was associated with a higher success rate than a self-expandable valve.29,30 A total of 241 patients were randomized to receive one or the other valve. Device success was achieved in 95.9% of the balloon-expandable valve group and in 77.5% of the groups with the self-expandable valve (P<.001) owing to a lower rate of residual aortic regurgitation (4.1% vs 18.3%; P<.001) and the less frequent need to implant more than 1 valve (0.8% vs 5.8%; P=.03) in the balloon-expandable valve group. Cardiovascular mortality at 30 days was similar (4.1% and 4.3%, respectively; P=nonsignificant) and there were no significant differences in the rates of bleeding and vascular complications, but placement of a permanent pacemaker was required more frequently in the self-expandable valve group (17.3% vs 37.6%; P=.001).
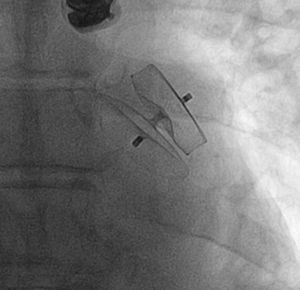
Percutaneous Left Atrial Appendage ClosureThe IBERIAN registry31 collected retrospective data on 167 patients with nonvalvular atrial fibrillation and a contraindication for anticoagulation therapy who underwent placement of a device in left atrial appendage. The mean age was 75 years and the procedure was successful in 95% of the patients (Figure 1). During the 2-year follow-up, 19% experienced an event of some type, with a decrease of 75% in the incidence of stroke with respect to the rate expected according to the risk score (2.4% vs 9.6%). Overall mortality was 11% and the rate of major bleeding was 6%.
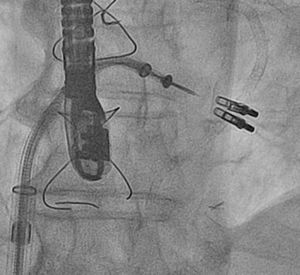
Percutaneous Mitral Valve RepairPercutaneous treatment of mitral regurgitation with addition of the MitraClip® system to medical treatment has been found to be safe and effective. A retrospective observational study reviewed all the patients treated with the MitraClip® in Spain between November 2011 and July 2013 in the 4 Spanish hospitals performing the highest numbers of implantations.32 The investigators included 62 patients with a mean (standard deviation) ejection fraction of 36% (14%), functional class III (37%) or IV (63%), and high surgical risk (EuroSCORE, 17 [11]). The device was successfully implanted in 98% (a single clip in 61%, 2 or more clips in 39%) (Figure 2). At the 1-year follow-up visit, 91% of the patients were in functional class ≤ II and mitral regurgitation was ≤ 2 in 81%. The 1-year mortality rate was 6.5%.
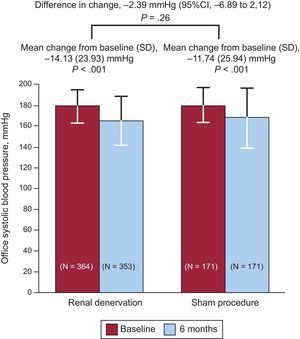
Renal DenervationThe SYMPLICITY HTN-3 trial,33 a controlled, randomized, prospective study involving a sham procedure, evaluated the efficacy of renal denervation in patients with resistant hypertension. The primary efficacy end point was the change in systolic blood pressure at 6 months and the secondary end point, the change in mean 24-hour ambulatory systolic blood pressure. The primary safety end point was a composite of death, renal disease, embolic events, hypertensive crisis, or new renal artery stenosis at 6 months. A total of 535 patients were randomized (2:1), and the mean decrease in systolic blood pressure at 6 months was 14.1 (23.9) mmHg in the denervation group and 11.7 (25.9) mmHg in the sham group (P<.001 for both vs baseline and P=.26 for superiority of denervation) (Figure 3). The change in mean 24-hour ambulatory systolic blood pressure was 6.8 (15.1) mmHg in the denervation group and 4.8 (17.3) mmHg in the sham group (P=.98 for superiority). There were no significant differences between the 2 groups in terms of safety.
Results of the primary end point (lower mean systolic blood pressure at 6 months) in the SYMPLICITY HTN-3 trial, which compared renal denervation with a sham procedure. 95%CI, 95% confidence interval; SD, standard deviation. Reproduced with permission from Bhatt et al.33
The treatment of elderly patients with acute coronary syndrome makes them a high-risk population because it is difficult to predict the risk of bleeding and the impact that frailty and their functional status may have on prognosis.34,35 The Swedish SCAAR registry36 confirmed the safety and efficacy of primary PCI in patients older than 80 years and demonstrated that the prognosis of those who survived the early phase after myocardial infarction was even slightly better than that of the general population. Advances in medical treatment and the increased use of the transradial approach may have contributed to this improvement in the outcome. With respect to the treatment of non—ST-segment elevation myocardial infarction, the contribution of Angeli et al37 demonstrated that elderly patients, who more frequently receive conservative or selectively invasive treatment, benefited even more than younger patients from early invasive management.
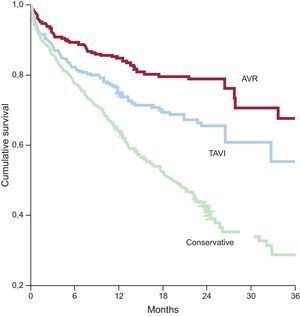
Aortic StenosisThe PEGASO registry38 demonstrated that, although most octogenarians with severe symptomatic aortic stenosis receive conservative treatment in Spain, their survival improved when they underwent intervention, whether percutaneous or surgical (Figure 4). The results of the US CoreValve trial,28 carried out in a population with a mean age of 83 years, suggest that the introduction of TAVI is even associated with an improvement in survival when compared with surgical aortic valve replacement. Importantly, this improvement was similar in patients both older and younger than 85 years of age. This result indicates that, although certain complications associated with TAVI, such as vascular events or conduction disorders, occur more frequently in elderly patients, TAVI is becoming—if it is not already— the treatment of choice for octogenarians with severe symptomatic aortic stenosis.39,40
Cumulative survival in the Prospective Registry of Symptomatic Severe Aortic Stenosis in Octogenarians. AVR, aortic valve replacement; TAVI, transcatheter aortic valve implantation. Adapted from Martínez-Sellés et al.38
On the other hand, it is essential that any octogenarian who considers undergoing TAVI be subjected to an integrated geriatric evaluation, since a number of simple scales for scoring dependence or frailty have been found to be useful for establishing the prognosis in these patients. A European registry is currently underway precisely to assess the usefulness of several of these parameters in the prediction of the post-TAVI prognosis.41 The idea is to change the current concept of “heart team” to one of “patient team”, which examines not only cardiovascular characteristics, but allows integrated patient evaluation.
CONFLICTS OF INTERESTNone declared.