Advanced lipoprotein phenotyping is a better predictor of atherosclerotic cardiovascular risk than cholesterol concentration alone. Lipoprotein profiling in heart failure (HF) is incompletely characterized. We aimed to describe the lipoprotein profile in patients with chronic HF compared with a matched control population.
MethodsThis cross-sectional study was performed from May 2006 to April 2014 and included ambulatory patients with chronic HF. Lipid concentrations and the size of main lipoprotein fractions (high-density lipoprotein [HDL], low-density lipoprotein [LDL], and very low-density lipoprotein) and the particle concentration of their 3 subfractions (large, medium and small) were assessed using 1H magnetic resonance spectroscopy.
ResultsThe 429 included patients with chronic HF were compared with 428 matched controls. Patients with chronic HF had lower total cholesterol and lower mean LDL (1115 vs 1352 nmol/L; P<.001) and HDL (25.7 vs 27.9μmol/L; P <.001) particle concentrations, with this last difference being mediated by a significantly lower concentration of the small subfraction of HDL (15.2 vs 18.6μmol/L; P <.001). Mean very low-density lipoprotein, LDL, and HDL particle size was significantly higher in patients with HF vs controls. All HDL-related differences from controls persisted after adjustment for New York Heart Association functional class or body mass index. We found strong negative correlations of known cardiac biomarkers (N-terminal pro-brain natriuretic peptide and interleukin-1 receptor-like 1) with total and small LDL and HDL fractions and HDL particle size.
ConclusionsPatients with chronic HF significantly differ in their lipoprotein profile compared with unaffected controls. Further research is needed to better understand the pathogenic relevance of this difference.
Keywords
Cardiovascular disease is the leading cause of morbidity and mortality in developed countries. Atherosclerotic-associated events such as myocardial infarction and ischemic stroke are increasing annually worldwide,1 and heart failure (HF) is also a growing cause of cardiovascular morbidity and mortality. In some regions, the prevalence of HF is predicted to increase by more than 50% by 2030.2
Consequently, individual cardiovascular risk stratification is recommended and typically assessed using classic lipid profile components such as total cholesterol, triglycerides, low-density lipoprotein (LDL) cholesterol (LDL-C), and high-density lipoprotein (HDL) cholesterol (HDL-C).3 However, this classic risk stratification accounts for atherosclerotic-mediated diseases but not for HF. Moreover, although high levels of LDL-C and low levels of HDL-C are known risk factors for future cardiovascular events, statin therapy does not abolish the total cardiovascular risk, even when LDL-C is normalized. In addition, attempts to reduce cardiovascular risk by increasing circulating levels of HDL-C have been unsuccessful.4–7 As a result, interest has focused on studying the detailed lipoprotein profile rather than total cholesterol, triglycerides, or LDL-C and HDL-C alone. Lipoprotein tests based on 2-dimensional (2D) diffusion-ordered 1H-magnetic resonance (MR) spectroscopy have emerged as novel tests for analyzing lipoprotein parameters beyond LDL-C, including particle concentration (-P), composition, and size.8,9 Indeed, LDL-P, HDL-P, and their subfractions have been described as better predictors of cardiovascular risk than LDL-C or HDL-C.10–13 However, studies so far have focused mainly on atherosclerotic risk and not in the HF population, and the few that have examined changes in the lipoprotein profile in HF have largely involved the HDL-P and HDL subfractions, globally suggesting that they are involved in risk of HF, especially when patients exhibit a decrease in small HDL particle subfraction.14–17 To date, knowledge is lacking on how the entire lipoprotein profile is affected in ambulatory patients with chronic HF.
The aim of our study was to describe the profile of a complete lipoprotein analysis using 2D diffusion-ordered MR spectroscopy in a population with chronic HF and to compare these findings with those in a clinically matched cohort without HF. To determine whether differences could also be detected with disease progression, we analyzed the correlation of the observed results with New York Heart Association (NYHA) class and classic cardiac biomarkers of inflammation, fibrosis and remodeling (interleukin-1 receptor-like 1 [ST2]), injury (high-sensitivity troponin T [hs-TnT]), and cardiac stretch (N-terminal pro-brain natriuretic peptide [NT-proBNP]).
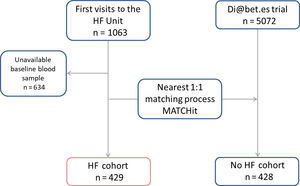
METHODSStudy population and designThis retrospective observational and comparative study, performed from May 2006 to April 2014, included ambulatory patients with chronic HF referred to a structured multidisciplinary HF unit at a large tertiary university hospital in the North-Western metropolitan area of Barcelona in Catalonia, Spain. Patients were referred to the HF unit mainly by the cardiology or internal medicine departments, and to a lesser extent by the emergency or other hospital departments. The criteria for referral to the HF unit were HF according to the European Society of Cardiology guidelines, at least 1 previous HF hospitalization, and/or reduced left ventricular ejection fraction.18 Patients were considered for the study regardless of HF etiology and were included if they were aged ≥ 18 years and had an available baseline blood sample.
Matched control cohortWe compared the data for the HF patients with those of control individuals recruited from the Dia@bet.es study,19 a national, cross-sectional, population-based study conducted from 2008 to 2010, designed to examine the prevalence of diabetes and impaired glucose regulation in Spain. This cluster sampling study included 5072 participants randomly selected from the entire Spanish region. We applied a nearest neighbor matching process (MATCHit technique20) to select the best control matches from the Dia@bet.es cohort for each individual in the case group by sex, age, body mass index (BMI), diabetes and statin therapy simultaneously, optimizing the overall distance among cases and matched controls. No control participants had previous cardiovascular disease. To make the cohorts more comparable, hypertension or dyslipidemia were not exclusion criteria.
During the baseline visit in both cohorts, patients provided written consent for use of their clinical data for research purposes. The study was approved by the respective local ethics committee (code: EO 10-076 and PI 13-095). The study was performed in compliance with the law protecting personal data and in accordance with the international guidelines on clinical investigation of the World Medical Association's Declaration of Helsinki.21
Lipoprotein analysis by 1H resonance spectroscopyBaseline blood samples were obtained in the fasting state between 09:00hours and 12:00hours and stored at –80°C without previous freeze-thaw cycles. A 250-μL aliquot of serum from each patient was shipped on dry ice to Biosfer Teslab (Reus, Spain) for lipoprotein analysis using the Liposcale Test.8 This advanced lipoprotein profile includes characterization of the lipid content (ie, cholesterol and triglycerides) and mean normalized size (-Z) of each lipoprotein class and -P of the large, medium, and small lipoprotein subfractions. Cutoff values were as follows:> 60nm, 45-60nm, and 35-45nm, respectively, for large, medium, and small very low-density lipoproteins (VLDL); 22.5-27nm, 20-22.5nm, and 18-20nm, respectively, for large, medium, and small LDL; and 9-13nm, 8.2-9nm, and <8.2nm, respectively, for large, medium, and small HDL. The variation coefficients for the -P ranged from 2% to 4% and were lower than 0.3% for particle sizes. The HDL-C/P ratio was calculated as an estimate of the cholesterol content of the HDL particles.17,22 To calculate the ratio, units of HDL-C were transformed to μmol/L.
Biomarker assaysNT-proBNP levels were determined using an immunoelectrochemiluminescence method (Elecsys; Roche Diagnostics, Switzerland), which had inter-run coefficients of variation of 0.9% to 5.5%. We measured hs-TnT levels using an electrochemiluminescence immunoassay with the Modular Analytics E 170 (Roche Diagnostics, Spain). The hs-TnT assay had an analytical range of 3 to 10 000 ng/L, and the coefficients of variation was 9% at the 99th percentile value of 13 ng/L. ST2 levels in plasma samples were measured using a high-sensitivity sandwich monoclonal immunoassay (Presage ST2 assay; Critical Diagnostics, United States), which had a within-run coefficient of <2.5% and a total coefficients of variation of 4%.
Statistical analysisContinuous variables are expressed as means±SD or medians [quartiles Q1–Q3] for normal and nonnormal distributions, respectively. The normality of continuous variables was assessed by Q-Q plots. Differences between groups were assessed by the Student t-test, analysis of variance, the Mann-Whitney U test, or the Kruskal-Wallis test, depending on the number of comparisons and the distributions of the quantitative variables. The significance of the differences in qualitative variables was assessed using the chi-square or Fisher exact test. Because the lipoprotein profile can vary with clinical status, comparison of cholesterol, triglycerides, and lipoprotein profile between HF patients and controls using BMI and NYHA functional class was also performed. An adjustment for multiple comparisons was performed by the Bonferroni technique. Spearman rank correlations were used to test the correlations of HDL and LDL parameters with demographic and clinical data, and with ST2, hs-TnT, and NT-proBNP. A 2-sided P <.05 was considered significant. All statistical analyses were performed using STATA V.13.0 (College Station, United States).
RESULTSPopulation characteristicsThe final study cohort consisted of 429 patients with chronic HF, with ischemic heart disease (47.8%), dilated cardiomyopathy (16.3%), and hypertensive cardiomyopathy (10%) as the most frequent etiologies. Results were compared with those for 428 control individuals (figure 1). Baseline characteristics of both cohorts are described in table 1. Both cohorts were mainly white (99.6%) and young-old (67.2 vs 66.3 years), with a high proportion of men (72.7% vs 68.5%). The cohort with chronic HF had a mean left ventricular ejection fraction of 35.5±14.4%, with NYHA class III-IV HF in 22%. In the patient cohort, mean ST2 was 51.2±39.9 ng/mL, mean hs-TnT was 44.6±62.3 ng/L, and mean NT-proBNP was 5393.1±18040.0 ng/L. The cohort with chronic HF also had a higher proportion of patients with hypertension (67.1% vs 46.9% in the control cohort; P <.001), whereas the control cohort had a higher proportion of participants with dyslipidemia (81.3% vs 65.0% in the HF cohort; P <.001). Patients with chronic HF had a lower mean BMI (27.3 vs 28.1kg/m2; P=.011) and worse overall renal function (estimated glomerular filtration rate, 54.0 vs 86.9mL/min/1.73 m2; P <.001).
Baseline patient characteristics
| Heart failure | Controls | P | |
|---|---|---|---|
| n=429 | n=428 | ||
| Age, y | 67.2±13.4 | 66.3±12.4 | .316 |
| Male sex | 312 (72.7) | 293 (68.5) | .170 |
| Diabetes | 194 (45.2) | 165 (38.5) | .051 |
| Hypertension | 288 (67.1) | 195 (46.9) | <.001 |
| Dyslipidemia | 279 (65.0) | 348 (81.3) | <.001 |
| Statins | 335 (78.1) | 338 (79.0) | .753 |
| BMI | 27.3±4.8 | 28.1±4.3 | .011 |
| eGFR, mL/min/1.73 m2 | 54.0 [36.4-81.1] | 86.9 [68.8-108.3] | <.001 |
BMI, body mass index; eGFR, estimated glomerular filtration rate calculated by Cockroft-Gault formula.
Unless otherwise indicated, the results are expressed as No. (%) for categorical variables or mean±standard deviation or median [25th-75th] percentiles for quantitative variables.
Total cholesterol, triglycerides, and a complete lipoprotein profile for each cohort are detailed in table 2. Patients with chronic HF had significantly lower serum total cholesterol (195.3 vs 215.4mg/dL among controls; P <.001), mainly because of lower LDL-C (110.6 vs 129.9mg/dL; P <.001) and HDL-C (48.6 vs 51.9mg/dL; P <.001). VLDL-C was significantly higher among patients with HF vs controls (19.0 vs 18.1mg/dL; P=.022). Total triglycerides did not differ between the 2 groups.
Comparison between cholesterol, triglycerides and lipoprotein subclasses between individuals with heart failure and controls
| Heart failure | Controls | P | |
|---|---|---|---|
| n=429 | n=428 | ||
| Total cholesterol, mg/dL | 195.29±37.86 | 215.39±34.59 | <.001 |
| Total triglycerides, mg/dL | 115.62 [92.67-149.05] | 115.66 [89.76-145.64] | .941 |
| VLDL particle number, nmol/L | |||
| Total | 51.75 [38.25-72.06] | 50.08 [34.96-72.27] | .735 |
| Large | 1.39 [1.09-1.74] | 1.40 [1.03-1.92] | .081 |
| Medium | 5.63 [4.14-7.54] | 4.20 [2.94-5.83] | <.001 |
| Small | 44.32 [32.82-62.98] | 43.11 [30.21-65.24] | .422 |
| VLDL-C, mg/dL | 19.04 [14.52-26.61] | 18.14 [11.74-24.82] | .022 |
| VLDL-TG, mg/dL | 69.58 [50.82-96.21] | 65.32 [46.25-92.24] | .935 |
| VLDL-Z, nm | 42.17±0.20 | 41.90±0.42 | <.001 |
| LDL particle number, nmol/L | |||
| Total | 1155±275.7 | 1352±283.9 | <.001 |
| Large | 179.8±38.0 | 182.3±39.8 | .347 |
| Medium | 339.9±125.2 | 377.6±135.7 | <.001 |
| Small | 635.4±137.3 | 792.2±176.0 | <.001 |
| LDL-C, mg/dL | 110.62±27.87 | 129.92±28.32 | <.001 |
| LDL-TG, mg/dL | 17.32±5.76 | 17.30±5.13 | .585 |
| LDL-Z, nm | 21.04±0.24 | 20.92±0.39 | <.001 |
| HDL particle number, μmol/L | |||
| Total | 25.68±5.43 | 27.86±4.84 | <.001 |
| Large | 0.29 [0.26-0.33] | 0.27 [0.25-0.30] | <.001 |
| Medium | 10.23±1.99 | 8.99±1.50 | <.001 |
| Small | 15.15±4.92 | 18.59±4.20 | <.001 |
| HDL-C, mg/dL | 48.57±10.41 | 51.91±11.07 | <.001 |
| HDL-TG, mg/dL | 16.61 [14.41-19.95] | 17.19 [14.28-20.49] | .585 |
| HDL-Z, nm | 8.32 [8.25-8.51] | 8.24 [8.19-8.31] | <.001 |
| HDL-C/P ratio | 49.26±5.89 | 47.99±4.00 | <.001 |
-C, cholesterol content; HDL, high-density lipoprotein; HDL-C/P, HDL cholesterol per HDL particle ratio; LDL, low-density lipoprotein; -TG, triglyceride content; VLDL, very low-density lipoprotein; -Z, mean normalized particle size.
The results expressed as mean±standard deviation or median [25th-75th] percentiles.
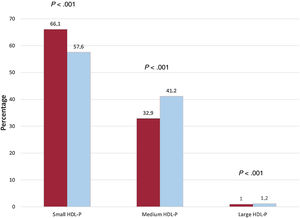
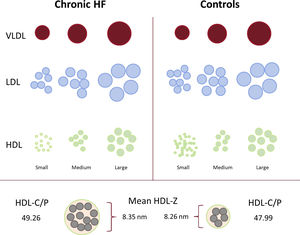
In the chronic HF population vs the controls, values were lower for overall LDL-P (1155 vs 1352 nmol/L) and HDL-P (25.7 vs 27.9μmol/L; both P <.001). Detailed subfraction analysis highlighted that the overall lower LDL-P was attributable to lower values for all 3 subfractions. The overall lower total HDL-P concentration was mainly the result of lower small HDL (15.2 vs 18.6μmol/L; P <.001); however, HDL medium and large subfractions were significantly higher in the cohort with chronic HF (10.23 vs 8.99 for medium and 0.29 vs 0.27μmol/L for large; P <.001). There was a relative negative difference of 8.5% for small HDL and relative positive differences of 8.3% for medium and 0.2% for large HDL (figure 2). No significant differences were observed in VLDL-P. Regarding lipoprotein particle size, all 3 (VLDL, LDL, and HDL) particles were larger in samples from patients with HF than in controls. Additionally, the calculated HDL-C/P ratio, an estimator of the degree of cholesterol content in the HDL particles, was higher in the chronic HF population (49.26 vs 47.99; P <.001) (figure 3). Differences in lipoprotein profile according to ischemic etiology were also assessed in a sensitivity analysis; this additional analysis yielded consistent results, with these differences being more accentuated in the ischemic group ().
Proportion of HDL lipoprotein subfractions in patients with chronic heart failure and matched controls. Comparison of differences in the proportion of small, medium, and large subfractions of HDL particles between chronic HF (orange) and controls (blue). HDL-P, high-density lipoprotein particle concentration; HF, heart failure. The chi-square test was used for comparisons.
Lipoprotein profile in patients with chronic HF vs matched controls. Schematic overview of the lipoprotein disruption observed in the chronic HF population compared with matched controls. Overall reduction in LDL-P and HDL-P particle concentration, with the latter being mediated by a significant decrease in the small subfraction of HDL. Overall increase in particle size, with an increase in cholesterol content of the HDL particles, expressed as an increase in the HDL-C/P ratio. HDL, high-density lipoprotein; HDL-C/P, HDL cholesterol per HDL particle ratio; HF, heart failure; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein; -Z, mean normalized particle size.
We found significantly lower total serum cholesterol concentrations across NYHA I-II and III-IV class patients compared with controls, with even lower concentrations of total cholesterol in those with III-IV NYHA class HF (table 3). Significantly lower concentrations of VLDL-C were found in patients with NYHA III-IV class HF, and lower concentrations of HDL-C were found in those with NYHA I-II class HF compared with controls. We also observed a trend to lower LDL-C with worsening NYHA functional class.
Comparison between cholesterol, triglycerides and lipoprotein subclasses between individuals with heart failure and controls according to NYHA class
| Heart failure | Controls | P | ||
|---|---|---|---|---|
| NYHA III-IV | NYHA I-II | |||
| n=93 | n=336 | n=428 | ||
| Total cholesterol, mg/dL | 191.5±40.3 | 196.3±37.1 | 215.39±34.59 | <.001a,b |
| Total triglycerides, mg/dL | 103.8 [90.1-135.2] | 118.3 [94.5-151.2] | 115.7 [89.8-145.6] | .207 |
| VLDL particle number, nmol/L | ||||
| Total | 44.14 [36.23-58.10] | 54.29 [39.37-73.93] | 50.08 [34.96-72.27] | .088 |
| Large | 1.27 [1.05-1.58] | 1.42 [1.11-1.80] | 1.40 [1.03-1.92] | .039 |
| Medium | 4.53 [3.65-6.57] | 5.90 [4.32-7.74] | 4.20 [2.94-5.84] | <.001a |
| Small | 38.33 [31.35-49.88] | 47.33 [33.43-64.51] | 43.11 [30.21-65.24] | .078 |
| VLDL-C, mg/dL | 17.13 [13.28-23.84] | 19.96 [15.23-27.76] | 18.14 [11.74-24.82] | .007a |
| VLDL-TG, mg/dL | 58.84 [48.29-79.09] | 72.97 [52.73-97.43] | 65.32 [46.25-92.24] | .083 |
| VLDL-Z, nm | 42.17±0.19 | 42.16±0.20 | 41.90±0.42 | <.001a,b |
| LDL particle number, nmol/L | ||||
| Total | 1128±280.3 | 1162±274.4 | 1352±283.9 | <.001a,b |
| Large | 180.1±38.4 | 179.7±37.9 | 182.3±39.8 | .640 |
| Medium | 345.0±131.3 | 338.5±123.6 | 336.6±135.7 | <.001a |
| Small | 603.6±136.2 | 644.1±136.5 | 792.2±176.0 | <.001a,b |
| LDL-C, mg/dL | 108.40±27.98 | 111.23±27.85 | 129.92±28.32 | <.001a,b |
| LDL-TG, mg/dL | 17.98±6.59 | 17.14±5.50 | 17.30±5.13 | .417 |
| LDL-Z, nm | 21.12±0.25 | 21.02±0.24 | 20.92±0.39 | <.001a,b,c |
| HDL particle number, μmol/L | ||||
| Total | 24.94±6.16 | 25.88±5.20 | 27.86±4.84 | <.001a,b |
| Large | 0.31 [0.27-0.35] | 0.30 [0.26-0.32] | 0.27 [0.25-0.30] | <.001a,b,c |
| Medium | 11.06±2.35 | 10.00±1.81 | 8.99±1.50 | <.001a,b,c |
| Small | 13.57±5.18 | 15.59±4.76 | 18.59±4.20 | <.001a,b,c |
| HDL-C, mg/dL | 49.04±11.78 | 48.44±10.01 | 51.91±11.07 | <.001a |
| HDL-TG, mg/dL | 16.29 [14.68-19.96] | 16.77 [14.27-19.87] | 17.19 [14.28-20.49] | .751 |
| HDL-Z, nm | 8.38 [8.32-8.47] | 8.31 [8.25-8.38] | 8.24 [8.19-8.31] | <.001a,b,c |
| HDL-C/P ratio | 51.27±5.70 | 48.70±5.83 | 47.99±4.00 | <.001a,b,c |
-C, cholesterol content; HDL, high-density lipoprotein; HDL-C/P, HDL cholesterol per HDL particle ratio; LDL, low-density lipoprotein; NYHA, New York Heart Association; -TG, triglyceride content; VLDL, very low-density lipoprotein; -Z, mean normalized particle size.
The results are expressed as mean±standard deviation or median [25th-75th] percentiles.
Significant values P <.05.
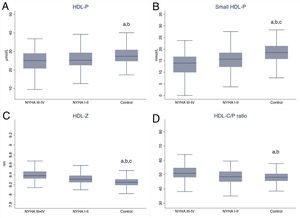
Total LDL-P and HDL-P was lower in patients with HF vs controls, with a significant downward trend with worsening NYHA functional class (P <.001). Like the entire cohort, these differences were mainly the result of significant differences in small subfractions, regardless of NYHA class severity, and were in keeping with those observed in the entire cohort with chronic HF. Lipoprotein particle size increased with worsening NYHA class, with VLDL-Z, LDL-Z, and HDL-Z the largest among the patients with NYHA III-IV class HF (P <.001).
The HDL-C/P ratio showed an increasing trend with worsening NYHA functional class and was highest in patients with NYHA III-IV class HF (P <.001) (figure 4).
HDL lipoprotein profile disruption across NYHA functional class in patients with chronic HF compared with a matched control cohort. Box plots comparing total HDL-P (A), small HDL subfraction (B), mean HDL particle size (C), and calculated HDL-C/P ratio (D) across NYHA functional class in HF patients vs matched controls with no HF. HDL cholesterol per HDL particle ratio; HDL-C/P, HDL cholesterol per HDL particle ratio; HF, heart failure; NYHA, New York Heart Association; -P, particle concentration; -Z, mean normalized particle size. Significant values P <.05:
a Controls vs NYHA I-II;
b Controls vs NYHA III-IV
c NYHA I-II vs III-IV.
BMI did not alter the pattern of differences in lipoprotein profiles between patients with chronic HF and controls ().
Correlations between LDL and HDL particle measures and clinical and biological parametersWe investigated correlations between HDL and LDL particles and other clinical and biological parameters in the HF population (table 4). Briefly, left ventricular ejection fraction showed a significant positive correlation with the total serum concentration and the small subfraction of HDL-P. Significant negative associations were observed for NT-proBNP, hs-TnT, and ST2 with total LDL-P and HDL-P. All 3 biomarkers showed a significant positive association with HDL-Z and a negative association with the small LDL subfraction, which was maintained for NT-proBNP and ST2 for the small HDL subfraction. Conversely, NT-proBNP and ST2 showed positive associations with the HDL-C/P ratio and LDL-Z.
Correlation between 1H-MR-spectroscopy LDL and HDL lipoproteins with clinical and biochemical parameters in HF patients-
| LVEF, % | NT-proBNP, ng/L | Hs-TnT, ng/L | ST2, ng/mL | |
|---|---|---|---|---|
| LDL particle number, nmol/L | ||||
| Total | 0.09(0.00;0.18) | −0.24(−0.33;−0.14)*** | −0.28(−0.40;−0.16)*** | −0.18(−0.29;−0.07)** |
| Large | 0.11(0.01;0.20)* | −0.11(−0.21;−0.01)* | −0.21(−0.32;−0.09)*** | −0.05(−0.15;0.05) |
| Medium | 0.11(0.03;0.20)* | −0.13(−0.22;−0.03)** | −0.19(−0.31;−0.06)** | −0.05(−0.15;0.06) |
| Small | 0.05(−0.04;0.14) | −0.32(−0.41;−0.24)*** | −0.32(−0.44;−0.20)*** | −0.30(−0.40;−0.19)*** |
| LDL-C, mg/dL | 0.09(−0.01;0.18) | −0.20(−0.30;−0.11)*** | −0.30(−0.41;−0.19)*** | −0.16(−0.27;−0.05)** |
| LDL-Z, nm | 0.08(−0.02;0.17) | 0.23(0.14;0.32)*** | 0.10(−0.03;0.23) | 0.24(0.14;0.35)*** |
| HDL particle number, μmol/L | ||||
| Total | 0.18(0.08;0.27)*** | −0.21(−0.31;−0.12)*** | −0.27(−0.39;−0.15)*** | −0.19(−0.30;−0.07)** |
| Large | 0.12(0.03;0.22) * | 0.02(−0.07;0.12) | −0.07(−0.20;0.06) | −0.02(−0.13;0.09) |
| Medium | 0.13(0.03;0.23)** | 0.26(0.16;0.35)*** | 0.07(−0.06;0.20) | 0.19(0.08;0.30)** |
| Small | 0.14(0.04;0.23)** | −0.34(−0.42;−0.25)*** | −0.32(−0.44;0.21) | −0.28(−0.39;0.18)*** |
| HDL-C, mg/dL | 0.15(0.06;0.25)** | −0.08(−0.17;0.01) | −0.24(−0.36;−0.12)*** | −0.09(−0.20;0.02) |
| HDL-Z, nm | −0.05(−0.14;0.05) | 0.41(0.33;0.49)*** | 0.29(0.18;0.41)*** | 0.35(0.25;0.45)*** |
| HDL-C/P ratio | −0.01(−0.10;0.09) | 0.29(0.20;0.38)*** | 0.05(−0.07;0.17) | 0.23(0.12;0.33)*** |
-C, cholesterol content; HDL, high density lipoprotein; HDL-C/P, HDL cholesterol per HDL particle; HF, heart failure; hs-TnT, highly-sensitive cardiac troponin T; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; ST2, interleukin-1 receptor-like 1; -Z, mean normalized particle size.
The results express Spearman rank correlation coefficients and 95% confidence intervals.
Level of significance:
This study provides a detailed description of a lipoprotein profile analyzed by 2D diffusion-ordered MR spectroscopy in a real-life cohort of outpatients with chronic HF compared with a cohort matched for age, sex, diabetes, and statin therapy. Our study indicates that significant derangements in lipoprotein profile are present in chronic HF. In this disease, serum total cholesterol concentrations were significantly lower than in controls, mainly because of lower LDL-C and HDL-C. Patients with chronic HF also had higher values of all 3 lipoprotein particle sizes (VLDL-Z, LDL-Z, and HDL-Z) and lower overall LDL-P and HDL-P, the latter because of a lower small HDL subfraction. Finally, the observed disruption was consistent regardless of NYHA functional class, with a trend to worsening with increasing classification.
Serum total cholesterol concentrations, LDL-C, and LDL-P were lower in the cohort with chronic HF. A possible explanation is that total cholesterol and LDL may have declined because of advanced chronic HF. The progressive decrease in total cholesterol concentrations as NYHA functional class worsened could align with this hypothesis. Moreover, low total and LDL cholesterol levels have been previously associated with a worse prognosis and higher overall mortality in patients with HF, regardless of etiology.23 However, this last explanation, known as the “cholesterol paradox,” although plausible, is usually associated with cachexia.24,25 In contrast, we found differences in cholesterol concentrations between patients with HF and controls in the overweight and obesity ranges of BMI but not with BMI <25. Alternatively, in vitro studies have suggested that circulating cytokines, acting as causative agents of increased mortality, may decrease lipoprotein particle and cholesterol levels by dampening hepatic lipoprotein production and increasing LDL receptor activity. If so, LDL would be considered an independent predictor of mortality and not only a marker of nutritional status.26,27
The overall lower LDL-P and HDL-P observed in the HF cohort, with the latter attributable to fewer small HDL-Ps, was also reported by Potočnjak et al. and Hunter et al.14–16 Although both authors used different methods to analyze small HDL particles, they correlated this finding with increase mortality in patients with acute HF and in a cath–lab-based HF population, respectively. Likewise, we have recently reported an association of lower levels of the small HDL subfraction with cardiovascular death in patients with HF.17 Here we observed a progressive change in these parameters with worsening NYHA functional class and a significant positive correlation of total HDL-P and small HDL subfraction with left ventricular ejection fraction. These findings, along with a negative correlation of these disruptions with NT-proBNP and ST2 levels, suggest a probable association not only with HF itself but also with the severity of the disease. This observation aligns with previous reports.15,28 Overall, these results strongly imply the importance of HDL in HF. Whether these derangements in HDL are simply a marker in HF or are relevant in the pathophysiological process remains to be elucidated. It should be considered that HDL particles may exert beneficial effects on overall myocardial function. Experimental reports have demonstrated that HDL may protect against ischemia and reduce myocardial remodeling.29 Moreover, small dense HDL particles may exert anti-inflammatory properties in endothelial cells and inhibit oxidized LDL-induced apoptosis.30
Regarding lipoprotein particle size, we observed a larger size of all 3 (VLDL, LDL, and HDL) particles in patients with chronic HF compared with the controls. Triglycerides usually drive changes in lipoprotein diameter, but the 2 groups had fairly similar plasma triglycerides, suggesting that the larger size is a specific feature of chronic HF. Overall lipoprotein particle size has been associated with atherosclerotic cardiovascular risk,31 but described as mainly mediated by more large HDL-P and small dense LDL-P,31,32 which does not concur with our observations. In our cohort with chronic HF, the larger amounts of LDL-Z and HDL-Z were secondary to the lower values for the small LDL and HDL subfractions, respectively. As for the HDL particle count, HDL-P size has previously been positively associated with overall cardiovascular risk,32 all-cause mortality,16 and cardiovascular death in HF.17 As with our findings for small HDL, we also observed a positive gradation of VLDL-Z, LDL-Z, and HDL-Z with NYHA functional class. Hunter et al.16 reported a similar positive association of HDL-Z with degree of left ventricular dysfunction, finding the smallest HDL-Z in patients without HF and the greatest for those with HF with reduced ejection fraction, while those with HF and preserved ejection fraction fell in between. Moreover, in this study, we observed a positive association of HDL-Z with cardiac biomarkers, emphasizing a probable relationship of HDL-Z with both the presence and severity of HF.
Previous studies have explored the HDL-C/P ratio as a surrogate of the cholesterol content.17,22,33 Evidence demonstrates that cholesterol-overloaded HDL-P is associated with the progression of carotid atherosclerosis in a disease-free population and with cardiovascular mortality in a chronic HF population. In the present study, we found that patients with chronic HF had cholesterol-overloaded HDL-P compared with controls. As for the HDL-P concentration, in the small subfraction of HDL and HDL-Z, the HDL-C/P ratio appeared to be important in terms of both the presence and the severity of the HF process. The cholesterol overloading of HDL-P in HF is not well understood, and whether it is secondary to an alteration in HDL remodeling, cholesterol transport/efflux of HDL or to an anomalous clearance of large HDL needs to be further explored. The detailed pathophysiological mechanism of this finding in HF should be investigated.
Prior evidence is controversial regarding the influence of the cholesterol content of the HDL particle and the particle number of HDL-C with cardiovascular risk in long-term follow-up and it could be explained by the complex physiopathological mechanisms involved in its metabolism.34 The question of whether all differences observed in our study in the HF population have a true impact on long-term cardiovascular outcomes deserves more attention in future research.
LimitationsThis study has some limitations. With the observational design, we can describe differences between lipoprotein profile in patients with chronic HF compared with unaffected controls, but we cannot draw definitive conclusions from the results, which can only be used to generate hypotheses. As the study participants with HF were selected from a specialized HF unit, these results cannot be extrapolated to other HF populations, such as those with acute HF or primary health care HF patients. Due to the long period of inclusion, we cannot exclude an influence on the results of the study induced by changes in the diagnosis and treatment of HF and dyslipidemia during this period. HDL exerts multiple functions such as cholesterol efflux, antioxidant, and anti-inflammatory activity35; indeed, HDL functionality has been demonstrated to be more predictive of impaired outcomes than HDL concentration for HF.36,37 We have not measured any parameters of HDL functionality and we could not establish differences between the HF and non-HF population in these parameters. Similarly, we did not measure nutritional or inflammatory parameters and we could not rule out their influence on the observed results. Numerous other variables could be used as matching criteria in control selection, and the observed differences could vary.
The strengths of our study are that total cholesterol, triglycerides, and lipoprotein profile are representative of real-life patients with chronic HF. The control group was selected by matching for several clinical variables, such as age, sex, diabetes, and statin therapy, which could all affect the lipoprotein test results, making the observed differences more likely to be exclusively secondary to HF. Moreover, because lipoprotein levels may vary with clinical status, a detailed subgroup comparison accounting for BMI and NYHA functional class is also described. The results could serve as reference values for chronic HF in future studies.
CONCLUSIONIn an outpatient population with chronic HF, significant derangements in serum concentrations of total cholesterol and several lipoprotein particles were found in comparison with a matched control group. A detailed description of each variable has been presented so that the findings could serve in future as reference values in chronic HF. Overall, the population with chronic HF had lower serum total cholesterol concentrations and lower LDL-P and HDL-P concentrations, with the HDL-P findings being mediated by a significantly lower small HDL subfraction. Lipoprotein particle size was significantly larger in chronic HF, and these patients also had an elevated HDL-C/P ratio. The final causes for the differences observed in lipoprotein profile in HF are probably multifactorial, and should be elucidated in future research.
Of note, all HDL derangements detected in the patients with chronic HF were consistent regardless of NYHA functional class or BMI, although with a significant trend to worsening with worsening NYHA functional class. This pattern suggests that HDL particles should be further interrogated in the setting of HF to better understand their pathogenic role.
FUNDINGThis work was supported by grants from Fundació La MARATÓ de TV3 (201502 and 201516 to A. Bayés-Genís., 201602-30-31 to N. Alonso and J. Julve); the Ministerio de Educación y Ciencia (SAF2014-59892 to A. Bayés-Genís); AdvanceCat (2014-2020 to A. Bayés-Genís); the Ministerio de Economía y Competitividad (MINECO) - Instituto de Salud Carlos III (ISCIII) (PI17-00232 to J. Julve, PI17-01362 to N. Alonso, PI15-00625 to D. Mauricio and RED2018-102799-T to J. Julve); Centro de Investigación Biomédica en Red Enfermedades Cardiovaculares. (CIBERCV, CB16/11/00403 Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas, (CIBERDEM, CB15/00071 and CB07/08/0016), an initiative from ISCIII, Spain, with cofunding from the European Regional Development Fund (ERDF) and ISCIII Miguel Servet II Program (CPII18/00004 to J. Julve).
AUTHORS’ CONTRIBUTIONSA. Teis, Germán Cediel, N. Amigó, J. Julve, D. Mauricio, N. Alonso, and A. Bayés-Genís contributed to the conception and design of the work. A. Teis, N. Amigó, E. Castelblanco, J. Ribalta, M. Guardiola, J. Franch, M. Bermúdez-López, P. Codina and J. Lupón contributed to data acquisition for the study. A. Teis and G. Cediel performed the analysis and interpretation of the data. All authors participated actively in drafting the manuscript and revising it critically and approved and agreed on the final version.
A. Teis and E. Castelblanco contributed equally to this article.
CONFLICTS OF INTERESTN. Amigó owns stock in Biosfer Teslab and has a patent related to the lipoprotein profiling described in the present manuscript. The remaining authors have nothing to disclose. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
- –
Lipoprotein particle analysis has emerged as a promising novel marker of atherosclerotic-based cardiovascular events. However, information regarding how lipoprotein particles vary in HF is lacking.
- –
This article provides a detailed description of cholesterol and lipoprotein particles in patients with chronic HF compared with a matched control group, serving as possible reference values for future HF studies. In summary, patients with chronic HF have reduced LDL and HDL particle concentrations, with the latter being mediated by a reduction in the small HDL subfraction, making HDL particles larger and richer in cholesterol content. This pattern suggests that HDL particles should be further interrogated in the setting of HF to better understand their pathogenic role.
We wish to acknowledge the nursing team that actively participated in the collection for the biobank.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.09.008