Final position of the neo-commissures is uncontrolled during transcatheter aortic valve implantation (TAVI), potentially hindering coronary access and future procedures. We aimed to develop a standard method to achieve commissural alignment with the ACURATE neo valve.
MethodsThe relationship between native and TAVI neo-commissures was analyzed in 11 severe aortic stenosis patients undergoing TAVI. Based on computed tomography analysis, an in silico model was developed to predict final TAVI commissural posts position. A modified implantation technique, accurate commissural alignment (ACA) and a dedicated delivery system were developed. TAVI implants were tested in 3-dimensional (3D) printed models and in vivo. Commissural misalignment and coronary overlap (CO) were analyzed.
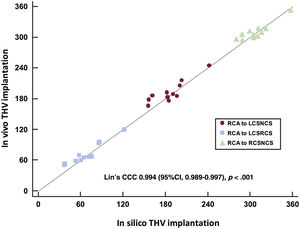
ResultsThe in silico model accurately predicted final position of commissural posts irrespective of the implantation technique performed (correlation coefficient, 0.994; 95%CI, 0.989-0.998; P<.001). TAVI implant with patient-specific rotation was simulated in 3D printed models and in 9 patients. ACA-oriented TAVI implants presented adequate commissural alignment in vivo (mean commissural misalignment of 7.7 ±3.9°). None of the ACA oriented implants showed CO, whereas in silico conventional implants predicted CO in 6 of the 9 cases.
ConclusionsAccurate commissural alignment of the ACURATE neo device is feasible by inserting the delivery system with a patient-specific rotation based on computed tomography analysis. This is a simple and reproducible method for commissural alignment that can be potentially used for all kinds of TAVI devices.
Keywords
The indications for transcatheter aortic valve implantation (TAVI) are evolving to include lower risk patients.1–4 With the longer life expectancy of TAVI recipients, procedural results should aim at matching the long-term benefits of surgical aortic valve replacement (SAVR) in all aspects. While commissural alignment is routinely performed in SAVR, the position of the prosthetic commissures is uncontrolled during TAVI.5 Since commissural posts may interfere with coronary ostia, a major concern is that these posts may hinder coronary access in future coronary interventions or favor coronary obstruction in an eventual TAVI-in-TAVI procedure.6 The magnitude of the problem is relevant since prior analyses have shown an incidence of CO that ranges between 31% and 51%,6 with only 22% of TAVI cases presenting adequate commissural alignment5 and, as recently reported by Barbanti et al., 7 in more than 7% of the cases post-TAVI coronary access might be unsuccessful. Methods to achieve commissural alignment are lacking for most of the available TAVI devices.
The accurate commissural alignment (ACA) project was designed to develop an implantation technique aiming to achieve native and neo-commissure alignment during the ACURATE neo TAVI procedure (Boston Scientific, United States). The present study aimed to verify the following hypothesis: a) the rotational behavior of the TAVI delivery system from femoral access to its final landing zone is predictable and related to the final orientation of the prosthesis in the aortic root; b) a controlled neo-commissural alignment of the ACURATE neo TAVI device can be achieved by planning a patient-specific rotation of the delivery system before advancing it within the vascular anatomy; and c) a dedicated user-friendly specifically developed device may help to achieve the intended final orientation of the TAVI device in the aortic root.
METHODSThe relation between native aortic commissures and transcatheter heart valve (THV) commissures was analyzed in 5 severe aortic stenosis patients undergoing TAVI with the ACURATE neo device in different scenarios. Scenarios differed according to: a) whether a conventional or modified THV implantation aiming to achieve commissural alignment was performed, and b) whether implantation was simulated (in silico or in printed 3-dimensional [3D] models) or in vivo. In 2 patients, TAVI implantation had been already done before the study design (conventional implantation group in silico and in vivo) and in 3 patients TAVI implantations were prospectively planned during the study course (modified implantation group in silico, in 3D printed models, and in vivo). Pre- and postprocedure computed tomography (CT) was available in all patients. Additionally, postprocedural CT was obtained in all the 3 modified implantations in printed 3D models. The ACA project was approved by the local ethics committee and patients provided informed consent.
Computed tomography acquisition and measurementsCT scans were acquired in a 128-row detector scan with a 0.625 mm slice thickness. Studies were contrast-enhanced, multiphasic and electrocardiographic gated; 70% of the cardiac cycle was selected for analysis. The orientation of THV commissural posts, native valve commissures and left coronary artery (LCA) were measured in a perpendicular cross-section multiplanar reconstruction of the aortic root as the clock-faded deviation angle starting at the right coronary artery (RCA) (0°), which was oriented at 12 o’clock, as previously described5,8 and as schematically depicted in figure 1. Measurements were performed from the center of the aorta or from the center or the THV stent frame when there was no full contact of the stent frame with the aorta. Afterwards the angle difference of each commissural post to its relative native commissure was measured, the mean commissure misalignment (CMA) was calculated for each implant as the mean difference of the prosthetic commissural post to native commissure. The result was classified as aligned (0-15°), mild CMA (15-30°), moderate CMA (30-45°), and severe CMA (45-60°). Significant CO of the commissural post in relation to the coronary artery ostia was defined as an angle equal or lower than 20°.
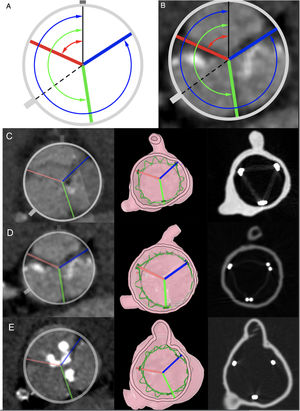
Cross-sectional images of pre-TAVI computed tomography scans summarizing the measurements for the evaluation of the degree of commissural misalignment. A, black line represents the right coronary ostium oriented at 12 o’clock; dashed line represents the left coronary ostium; blue line represents commissure between noncoronary sinus and right coronary sinus; red line represents the commissure between left coronary sinus and right coronary sinus; green line represents the commissure between left coronary sinus and noncoronary sinus. The arrows represent the 3 angles defined by right coronary and each commissure. B, previous scheme overlaid in a cross-sectional pre-TAVI scan. C, D* and E, pre-TAVI computed tomography scans showing the native aortic valve commissures (column 1), in silico (column 2) and in vitro (column 3) post-TAVI simulated implant with neo-commissural alignment, for cases 3, 4, and 5, respectively. *In silico ACURATE neo stent frame is represented in green.
We used pre- and post-TAVI CT scans of patients included in the ACA project in our institution. The final position of the implanted THV with the standard technique was simulated based on CT analysis performed before and after TAVI procedures with conventional implantation of the ACURATE neo valve in 2 patients. Then, the ACA orientation was prospectively estimated for 3 patients based on pre-TAVI CT. CT-derived aortic biomodels of the patients where obtained using Slicer.9 The centerline of the aorta, from the descending aorta to the aortic root and left ventricular outflow was then calculated. The biomodel meshes and centerlines were imported to a computer assisted design software (Rhinoceros, MCneel and Associates, United States) where further analysis was performed.
Stage 2: Bench testing in 3D printed modelsThe estimated required rotation of the delivery system to achieve a correct alignment of the neo-commissures with the native commissures by in silico simulation (ACA orientation) was used in bench testing implants of the ACURATE neo device in 3D printed models (printed in our VAL 3D LAB) and CT scans of the models once the valve was implanted were performed to demonstrate the accuracy of the prediction. Hollowed 3D printed-phantoms were obtained from pre-TAVI CT scans of 3 patients. This 3D-phantoms were intended to simulate TAVI procedures with angiographic guidance and were made in a multilateral fashion with a flexible silicone-like material (formlabs elastic, Formlabs Inc, United States) for aortic root and leaflets to allow a more realistic behavior during TAVI.
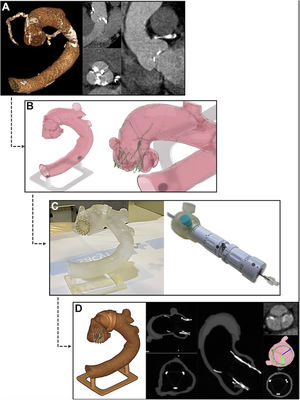
A dedicated tool was developed to accurately control the axial rotation of the TAVI delivery system (ACA delivery system, patent application number: P202030860). This tool is just a helpful element for measuring the degrees of rotation of the delivery system and consists of a circular element attached to the handler. ACURATE neo valves were implanted in the 3D-printed models with their predicted ACA orientation that was calculated as described for the in silico methods. After each implant, angiographic projections were recorded and CT scans of the 3D-printed model with the implanted valve were obtained for in silico vs in vitro comparison of commissural post orientation. The degree of deviation of in silico vs in vitro implants with ACA orientation were analyzed and are reported as the difference of the angles between the RCA and each commissural post in silico vs in vitro. This ACA oriented TAVI simulation protocol is described in figure 2.
Step-by-step summary of the calculation of accurate neo-commissural alignment orientation before the in vivo procedure. A, pre-TAVI computed tomography images. B, in silico simulated prosthesis implant with accurate commissural alignment. C, in vitro implant with accurate commissural alignment in a 3D printed model from the patient by rotating the delivery system as estimated in silico and assisted with the dedicated rotational tool. D, computed tomography scan of 3D printed model after prosthesis implantation verifying the achievement of accurate commissural alignment as intended.
In vivo implants with ACA orientation were performed in 9 consecutive patients. First, after successful TAVI implants in the 3D printed models based on pre-TAVI CT scans from 3 patients, the procedures were performed in vivo in the 3 of them with ACA orientation deployment of the ACURATE neo valve. The rotation of the delivery system–facilitated with the ACA delivery system–was the same in each patient-specific 3D-printed phantom. After TAVI implants angiographic projections were recorded comparison of for valve commissural posts with their corresponding 3D-printed model. Also, post-TAVI CT was performed in all cases to obtain a precise comparison of valve orientation in vivo vs in silico and in vitro simulations. Secondly, 6 more cases (cases 6 to 11) were treated with ACA orientation implant without previous in vitro simulation. Post-TAVI measurements were calculated using similar coregistration techniques based on the commissural post orientation at fluoroscopy as described elsewhere.6
Statistical analysisDifferences among in silico, in vitro and in vivo were calculated taking as reference the native commissure orientation in each case and are presented as mean±standard deviation. The degree of agreement between the different measures was obtained through Lin's concordance correlation coefficient taking each of the measurements as independent. Statistical analyses were performed using R 3.6.3 (R Fondation for Statistical Computing, Austria) and MedCalc 13.3.3 (MedCalc Software bvba, Belgium).
RESULTSIn silico simulationFirst, the hypothesis that axial rotation of the TAVI system when advanced along the aorta is related to the aortic centerline and remains perpendicular to the aortic centerline was demonstrated as shown in figure 3. The relationship of the prothesis within the aortic root, the patient-specific anatomic orientation of the Valsalva sinus, and coronary ostia location were estimated. A 3D model of an ACURATE neo valve was imported to the computer aid design software and oriented to the aortic centerline at the descending aorta with one commissural post aligned at 12 o’clock. This virtual prosthesis was then advanced up to the aortic valve plane obtaining the predictable position at its landing zone in a conventional-oriented implantation.
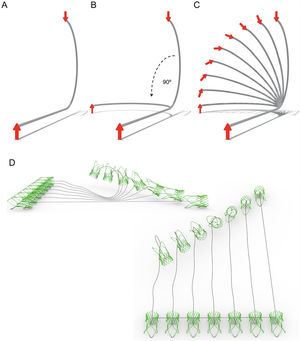
Simplified model of the aorta to predict TAVI system rotation along the aorta. A, simulation with no latero-lateral bends; when an arrow is advanced pointing toward 12 o’clock it will arrive at 6 o’clock to the distal end of the curve. B, if a later bend of 90° is added. When the arrow is again advanced pointing at 12 o’clock it will arrive at the distal end pointing at 12 o’clock. C, simulation of the rotational effect of the system with alternative in-between lateral bends. D, when the arrow is replaced in the model by a simulated prosthesis, the same principle is maintained.
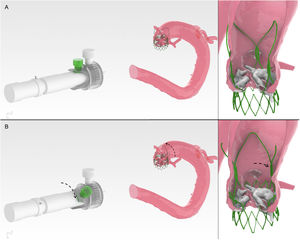
Then, the optimal rotation of the valve for achieving a commissure aligned implant was estimated using a self-developed script that allows a controlled rotation of the 3D virtual valve. A patient-specific clockwise or counterclockwise rotation was performed so that the commissural posts of the prothesis matched the native commissures in the aortic root biomodel, this optimal commissural aligned orientation was considered “the ACA orientation” (figure 4 and ).
The predicted orientation of the valve correlated well with the final implantation position of conventional ACURATE neo valve (with a commissural post pointing toward 12 o’clock) implant showing a mean difference between the post's simulated and actual position of 6.1±5.2°. Specific measurements for the retrospective cases are shown in . In addition, the degree of misalignment and CO are summarized in table 1.
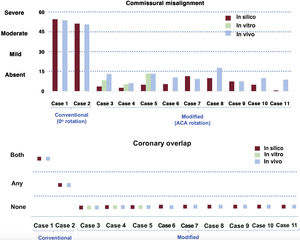
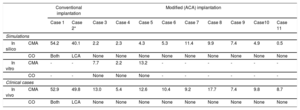
Degree of commissural misalignment and coronary overlap in the simulations (in silico and in vitro) and following in vivo procedures according to conventional vs modified (accurate commissural alignment) implantation.
| Conventional implantation | Modified (ACA) implantation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2* | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case10 | Case 11 | ||
| Simulations | ||||||||||||
| In silico | CMA | 54.2 | 40.1 | 2.2 | 2.3 | 4.3 | 5.3 | 11.4 | 9.9 | 7.4 | 4.9 | 0.5 |
| CO | Both | LCA | None | None | None | None | None | None | None | None | None | |
| In vitro | CMA | - | - | 7.7 | 2.2 | 13.2 | - | - | - | - | - | - |
| CO | - | - | None | None | None | - | - | - | - | - | - | |
| Clinical cases | ||||||||||||
| In vivo | CMA | 52.9 | 49.8 | 13.0 | 5.4 | 12.6 | 10.4 | 9.2 | 17.7 | 7.4 | 9.8 | 8.7 |
| CO | Both | LCA | None | None | None | None | None | None | None | None | None | |
Mean CMA expressed in degrees; CO expressed as none, RCA or LCA, or both. ACA, accurate commissural alignment; CMA, commissural misalignment; CO, coronary overlap; LCA, left coronary artery; RCA, right coronary artery.
The concordance of the commissural post orientation in the in silico THV implants to the final commissural post orientation was high, both in conventional and ACA implants (figure 5), with a Lin's concordance correlation coefficient of 0.994 (95%CI, 0.989-0.997).
Correlation of commissural post orientation in silico vs in vivo according to post-TAVI computed tomography scan. 95%CI, 95% confidence interval; CCC, concordance correlation coefficient; LCSNCS, commissural post between left coronary sinus and noncoronary sinus; LCSRCS, commissural post between left coronary sinus and right coronary sinus; RCA, right coronary artery; RCSNCS, commissural post between right coronary sinus and noncoronary sinus; THV, transcatheter heart valve.
Following this estimation of the ACA orientation, the required patient-specific rotation to achieve the ACA implant was: 60°, 35° and 45° anticlockwise for cases 3, 4 and 5, respectively (). The differences in the orientation of the commissures and LCA in the native valve, the in silico, the in vitro and the in vivo implants (ACA orientation) are reported in . The mean CMA in vitro for the 3 prospective cases was 8.2±3.3° (case 3), 5.2±6.6° (case 4), and 13.3±1.9° (case 5).
In vivo validationNone of the 9 ACA oriented cases presented significant coronary ostia overlap; in contrast, based on in silico simulated conventional implants, 6 out of 9 cases would have had CO of one or both coronary arteries; in case 6 the valve was correctly aligned at 0° of rotation, meaning that the ACA and the conventional implant were the same. In cases 3, 4 and 5 the needed rotation of the delivery system for ACA implant was the same as for the in vitro implant; in cases 6 to 11 the delivery system was rotated: 0°, clockwise 55°, anticlockwise 20°, anticlockwise 25°, clockwise 60°, and clockwise 40°, respectively. The THV were accurately rotated with the ACA device, before entering through the introducer. This way, torsion forces, which would have appeared if the rotation had been performed inside the patient, were avoided.
The incidence of CO and the degree of CMA are summarized in and figure 6. The entire process from in silico to in vivo ACA oriented implantation of an ACURATE neo is summarized in figure 7. A total of 8 out of 9 ACA implants showed commissural alignment; only case 8 showed mild CMA (mean CMA of 17.7±12.3°).
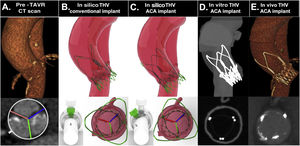
Case analysis and result after accurate commissural alignment from simulation to in vivo. A, native aortic valve in baseline computed tomography scan; B,C, in silico conventional and ACA oriented implant; D, computed tomography scan of 3D printed model with ACA oriented implant; E, computed tomography scan following ACA oriented ACURATE neo prosthesis implant. ACA, accurate commissural alignment; CT, computed tomography; pre-TAVR CT scan, computed tomograhy performed for plannification of transcatether aortic valve replacement; THV, transcatheter heart valve.
TAVI procedure, unlike SAVR, in which native leaflets are removed and the prosthetic valve is implanted maintaining native valve commissures’ orientation, does not consider the commissural alignment of the device. There is a suspected—and still under research—clinical benefit of such alignment, but no standard strategy to achieve this has yet been developed. Some authors advocate the rotation of the delivery system within the aorta, but such a strategy is imperfect and not exempt from risks. This prospective project aimed to explore the possibility of preprocedural planning of accurate commissural alignment and the main findings are as follows: a) the rotational orientation of the THV system within the aortic root during the conventional TAVI procedure is predictable; b) therefore, the required rotation of the THV delivery system to achieve a patient-specific commissural alignment can be easily estimated from the baseline CT scan; and c) the ACA of the ACURATE neo THV is feasible by simple rotation of the delivery system and, in particular, can be performed accurately with the ACA delivery system.
Future analysis of the clinical impact of commissural alignment compared with conventional TAVI implantation requires a systematic strategy to be reproducible. This is the first description of such a strategy and might allow analysis of the impact of the ACA on short-, mid-, and long-term outcomes. More likely, this strategy will become part of the standard planning of TAVI procedures if demonstrated in larger series given the potential benefits and its lack of risks. Moreover, the use of this technique might allow modification of current TAVI devices to reduce the radial force at the landing zone over the conduction system, potentially diminishing the need for a permanent pacemaker.
Coronary access in patients harboring a transcatheter aortic valve implantation deviceThe longer life expectancy of low-risk patients will increase the likelihood of developing or worsening pre-existing coronary artery disease requiring coronary angiography and percutaneous coronary intervention. Although practical guides for coronary access after TAVI are available,10,11 the time for a percutaneous intervention during an acute coronary syndrome after TAVI is much prolonged and, in between 3% and 9% of patient, access can be impossible.7,12 Low-profile THVs, such as the balloon expandable THV, allow easier coronary access than the larger self-expanding devices, whose design extends above the sinotubular junction. In the RESOLVE registry, Ochiai et al.12 described the incidence of unfavorable features for coronary catheterization, present in 25.8% for RCA and of 34.8% for LCA in patients harboring an Evolut Pro/Evolut R device compared with 8.1% and 15.7% for SAPIEN-3. Interestingly, the presence of these unfavorable features led to a significantly lower rate of successful coronary engagement for both devices.
Among 100 ACURATE neo conventional implants, Tang et al.6 reported an incidence of severe coronary overlap (CO) of one or both coronary arteries of 51%. A relatively intuitive orientation of the commissural posts in the coplanar cusp view (orienting them toward the inner curve of the aorta) reduced the incidence of CO to less than 15%. However, this was only performed in 7 cases and although the coronary obstruction rate was reduced, it persisted in one third of the patients and involved a manipulation of the delivery system in the ascending aorta, which might increase embolic risk. These manipulations of the delivery system intended to reduce the rate of nonocclusive coronary obstruction have also been tried successfully with alternative devices such as Evolut THV but, again, with moderate success and potential complications. In addition, no relationship was found between the clock-faded orientation of aortic root anatomic structures or coronary arteries with CO, although patients with CO tended to have a greater aortic root angle.
The ability to individualize the rotation of the THV before the system is introduced in the patient is an attractive hypothesis tested successfully in our investigation, and it might be of special interest in cases of anomalous location of the coronary ostium, such as cases of bicuspid aortic valves with coronary ostia separated by 180°.
Long-term prognosis and THV durabilityTAVI redo will become a more frequent scenario with the increasing number of TAVI procedures and the longer life expectancy of low-risk patients. Maneuvers to maximize THV durability are crucial and are currently focused on reducing leaflet stress by achieving better residual gradients and minimizing the residual aortic regurgitation,5,8,13 together with dedicated treatments during manufacturing to prevent calcification of the leaflets.14 Deformation and stress of the THV leaflets could be reduced by better alignment of the commissures according to prior research.13 In addition, Rogers et al.15 reported that up to 21% of patients have the leaflets sutured within the stent frame above the sinotubular junction, resulting in an increased risk for coronary obstruction during TAVI-in-TAVI as they may seal the stent frame, hindering the flow through the cells to the coronary ostia. Laceration of TAVI leaflets with transcatheter electrosurgery (BASILICA technique) has been reported to reduce coronary occlusion risk in valve-in-valve procedures.16 However, this complex strategy might be ineffective if there is a misalignment of the commissures, as the laceration of the leaflet would be central whereas the coronary artery ostium would not. Hence, several future problems could be prevented if an accurate alignment of the neo-commissures were performed systematically.
The main limitation of this proof-of-concept research is the small number of cases included. However, the accuracy of the measurement performed lays the basis for future multicenter studies reproducing this methodology on a larger scale. Prospective studies with ACURATE neo and alternative devices are needed to validate this hypothesis. CT measurements have been performed as described in the methods section; however, if larger scale research is performed, interobserver variability could be a source of bias. The impact of extreme tortuosity of other anatomical variations might have an impact on the final orientation of the TAVI device, and dedicated research is warranted for such cases.
CONCLUSIONSOur results suggest that an accurate commissural alignment of the ACURATE neo TAVI device is feasible by inserting the THV delivery system with a patient-specific rotation (ACA orientation) based on CT analysis. This systematic methodology minimizes the risk for the patient and will allow a safe comparison of clinical outcomes between conventional and ACA oriented strategies.
FUNDINGThe institution received unconditioned grants from Boston Scientific, Medtronic, Meril Life, and Abbott.
AUTHORS’ CONTRIBUTIONSA. Redondo, F. Valencia, and I. J. Amat-Santos designed the project. A. Redondo and I. J. Amat-Santos collected and analyzed the information and wrote the final manuscript. S. Santos-Martínez, J. R. Delgado-Arana, and A. Barrero helped to collect data. F. Valencia, A. Serrador, H. Gutiérrez, I. Sánchez-Lite, T. Sevilla, A. Revilla, C. Baladrón, W-K. Kim, M. Carrasco-Moraleja, and J. A. San Román performed a critical review and approved the final version of the manuscript.
- -
The final position of the neo-commissures is uncontrolled during TAVI, potentially hindering coronary access and future procedures. Several strategies have been developed for commissural alignment. For some devices, a standard orientation is recommended (not patient-specific and therefore imperfect); for others, manipulation of the delivery system within the patient might achieve good alignment but at the cost of increasing the risk of vascular damage or debris embolization. No patient-specific strategy has been developed to date.
- -
Accurate commissural alignment of the ACURATE neo device is feasible by inserting the delivery system with a patient-specific rotation based on CT analysis through an easy estimation of the final position of the valve. The delivery system can therefore be oriented before being introduced into the patient and good alignment of commissures with a lack of coronary overlap can be achieved. This is a simple and reproducible method for commissural alignment that can potentially be used for all kinds of TAVI devices.
I.J. Amat-Santos and W.K. Kim are both proctors for Boston Scientific.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.02.004