Calcific aortic valve disease (CAVD) is a leading cardiovascular disease in the elderly, which results in the failure of valvular function.1 Endothelial dysfunction, inflammation, and oxidative and mechanical stress lead to valve and myocardial remodeling, although mechanosensory pathways that promote calcific changes are yet to be identified. Integrin-linked kinase (ILK) is a key protein that regulates vascular tone and cardiac contractility, acting as a mechano-transducer of hemodynamic forces in the myocardium.2
We previously found that ILK expression in endothelial cells plays a pivotal role in regulating vasomotor tone by preventing uncoupling of endothelial nitric oxide synthase (eNOS). We described a disruption of this association with a clear correlation between ILK inhibition and atherosclerosis.3 As part of the inflammatory response, cytotoxic nitric oxide (NO) levels from inducible nitric oxide synthase (iNOS) promote endocytosis of ILK followed by lysosomal degradation, leading to atherosclerotic progression.4
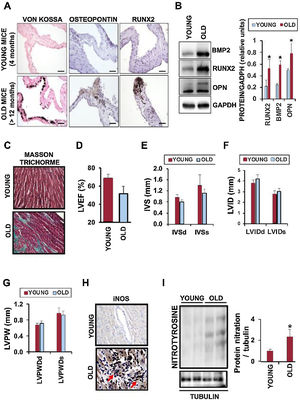
Endothelial dysfunction and atherosclerosis are 2 complications of aging in which ILK plays a significant role by still unexplored mechanisms. To test whether ILK may regulate CAVD, we used young and aged mice expressing ILK. Von Kossa staining of aortic valves from aged mice exhibited extensive calcium deposition compared with young animals, together with expression of osteogenic proteins osteopontin and Runx2 (figure 1A-B), with high levels of bone morphogenetic protein 2 (BMP2) (figure 1B). Aged hearts showed extensive fibrosis (figure 1C), but neither left ventricle diastolic nor systolic dysfunction, although end systolic interventricular septum thickness and left ventricular ejection fraction were reduced when compared with those in young mice (figure 1D-G).
Aging-related calcification of aortic valves. A: Von Kossa and immunohistochemical detection of osteopontin and Runx2 in aortic valves from young and old mice. B: immunoblot detection of BMP2, Runx2, and osteopontin in the aortic valves (n=4/group; mean±standard deviation; asterisk indicates P <.05 young vs old). C: Masson-trichrome staining of heart sections from the same hearts; left ventricular ejection fraction (D), end diastolic and systolic interventricular septum thickness (E), end diastolic and systolic left ventricular diameter (F), end diastolic and systolic left ventricle posterior wall thickness (G), expression of iNOS (H), and detection of protein nitration with antinitrotyrosine antibody in the hearts (I); n=4/group; mean±SD; asterisk indicates P <.05 young vs old.
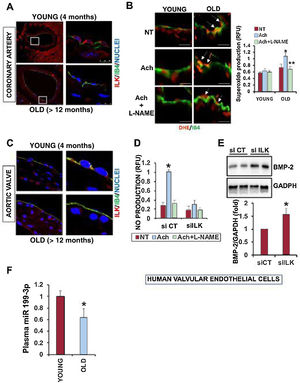
Protein nitration, indicative of nitrative stress, as result of high levels of cytotoxic NO from increased iNOS expression and superoxide anion formation, were found in the hearts of aged mice (figure 1H-I), and endothelial ILK was significantly reduced in the coronary arteries compared with levels in young adults (figure 2A). Accordingly, acetylcholine stimulation of murine coronary arteries from aged mice lead to eNOS-dependent superoxide generation, instead of endothelial derived NO, as inhibition of eNOS with L-NG-nitroarginine-methyl ester (L-NAME) was sufficient to efficiently revert this effect (figure 2B).
Expression of ILK in the hearts of young and old mice. A: left, confocal microscopy detection of endothelial ILK (Alexa 488, red) in coronary arteries; IB4 (fluorescein isothiocyanate (FITC), green, as endothelial marker). Right, magnified sections (open boxes), n=4/group. B: confocal microscopy detection of anion superoxide with dihydroethidium (DHE) fluorescence (red) in coronary arteries stimulated with 10μM acetylcholine (Ach), 30minutes or in combination with 500μM of nitric oxide synthase (NOS) inhibitor L-NAME; n=4/group. Mean±SD; asterisk indicates P <.05 Ach young vs old; double asterisk indicates P <.001 old Ach vs Ach plus L-NG-nitroarginine-methyl ester [L-NAME]). C: confocal microscopy detection of ILK (red) in the valves of the same mice; n=4/group. D: NO production in human valvular endotelial cells stimulated as in B, in which the expression of ILK was reduced by RNA interference (siILK). A non ILK siRNA (siCT) was used as a negative control (n=3; mean±SD; *P <.05 Ach vs L-NAME). E: expression of BMP2 in the same cells; n=3; mean±SD; asterisk indicates P <.05 siCT vs siILK). F: plasma levels of miR199-3p analyzed by RT-qPCR; n=4; mean±SD; asterisk indicates P <.05 young vs old.
As in the coronary arteries, endothelial ILK levels found in the aortic valves from aged mice were also lower than those in young adults (figure 2C). The causative role of ILK in valvular calcification was assayed in human valvular endothelial cells, in which ILK expression was decreased by transfecting with a specific small (si) interfering RNA (siILK). ILK silencing inhibited NO production by human valvular endotelial cells (hVECs) (figure 2D) and correlated with an increased expression of BMP2 (figure 2E), as in the aortic valves of aged mice (figure 1B).
We analyzed the expression of several microRNAs (miRNAs) involved in CAVD.5 Out of 11 miRNAs differentially expressed in young vs aged mice, miR 199-3p was related to the expression of Notch and eNOS, both genes directly related to CAVD (Notch) and vascular tone (eNOS). Silencing of Notch-1 increased the levels of Runx2, promoting valvular calcification. We unexpectedly found a correlation between downregulation of ILK with decreased levels of miR-199-3p in aged mice (figure 2F), suggesting a Notch-independent mechanism of aortic calcification by ILK, although Notch in CAVD is under debate, since it also promotes pro-osteogenic responses in human aortic valve interstitial cells.6
In conclusion, we found for the first time a significant association between decreased endothelial ILK, eNOS uncoupling and valvular calcification in aged mice, suggesting that ILK may prevent valvular calcification through miRNA199-3p. Further studies including the use of endothelial-specific conditional knockout mouse models will be crucial to validate the specific contribution of ILK and surrogated miRNAs as targets in CAVD.
All animal procedures were approved by the University of Alcalá Animal Care Committee and Autonomous Community of Madrid (experimental procedure 231.2/20) and conformed to the EU Directive on the protection of animals used for experimental and other scientific purposes (enacted under Spanish law 1201/2005).
FUNDINGThis work was supported by grants from the Spanish Society of Cardiology project for Basic Research in Cardiology in 2019 to P. Reventún, C. Zaragoza, M. Saura, and J.L. Zamorano, and by Junta de Comunidades de Castilla La Mancha, Ayudas a proyectos de investigación, cofinanced by European Regional Development Funds “European Union. A way of making Europe” (SBPLY/19/180501/000055), to M. Saura. A. Cook is a recipient of a University of Alcalá predoctoral research fellowship.
AUTHORS’ CONTRIBUTIONSS. Sánchez: experimentation, data acquisition. A. Cook: experimentation, data acquisition. P. Reventún: experimentation, data acquisition. C. Zaragoza: experimentation, review, editing. J. L. Zamorano: review, editing, funding acquisition. M. Saura: conceptualization, methodology, experimentation, original draft preparation, writing, review, editing, funding acquisition.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.


![Expression of ILK in the hearts of young and old mice. A: left, confocal microscopy detection of endothelial ILK (Alexa 488, red) in coronary arteries; IB4 (fluorescein isothiocyanate (FITC), green, as endothelial marker). Right, magnified sections (open boxes), n=4/group. B: confocal microscopy detection of anion superoxide with dihydroethidium (DHE) fluorescence (red) in coronary arteries stimulated with 10μM acetylcholine (Ach), 30minutes or in combination with 500μM of nitric oxide synthase (NOS) inhibitor L-NAME; n=4/group. Mean±SD; asterisk indicates P <.05 Ach young vs old; double asterisk indicates P <.001 old Ach vs Ach plus L-NG-nitroarginine-methyl ester [L-NAME]). C: confocal microscopy detection of ILK (red) in the valves of the same mice; n=4/group. D: NO production in human valvular endotelial cells stimulated as in B, in which the expression of ILK was reduced by RNA interference (siILK). A non ILK siRNA (siCT) was used as a negative control (n=3; mean±SD; *P <.05 Ach vs L-NAME). E: expression of BMP2 in the same cells; n=3; mean±SD; asterisk indicates P <.05 siCT vs siILK). F: plasma levels of miR199-3p analyzed by RT-qPCR; n=4; mean±SD; asterisk indicates P <.05 young vs old. Expression of ILK in the hearts of young and old mice. A: left, confocal microscopy detection of endothelial ILK (Alexa 488, red) in coronary arteries; IB4 (fluorescein isothiocyanate (FITC), green, as endothelial marker). Right, magnified sections (open boxes), n=4/group. B: confocal microscopy detection of anion superoxide with dihydroethidium (DHE) fluorescence (red) in coronary arteries stimulated with 10μM acetylcholine (Ach), 30minutes or in combination with 500μM of nitric oxide synthase (NOS) inhibitor L-NAME; n=4/group. Mean±SD; asterisk indicates P <.05 Ach young vs old; double asterisk indicates P <.001 old Ach vs Ach plus L-NG-nitroarginine-methyl ester [L-NAME]). C: confocal microscopy detection of ILK (red) in the valves of the same mice; n=4/group. D: NO production in human valvular endotelial cells stimulated as in B, in which the expression of ILK was reduced by RNA interference (siILK). A non ILK siRNA (siCT) was used as a negative control (n=3; mean±SD; *P <.05 Ach vs L-NAME). E: expression of BMP2 in the same cells; n=3; mean±SD; asterisk indicates P <.05 siCT vs siILK). F: plasma levels of miR199-3p analyzed by RT-qPCR; n=4; mean±SD; asterisk indicates P <.05 young vs old.](https://static.elsevier.es/multimedia/18855857/0000007500000001/v1_202112220611/S1885585721001869/v1_202112220611/en/main.assets/thumbnail/gr2.jpeg?xkr=eyJpdiI6IldRTktKSEtWY3hwVlQ1RDBJU0NWWnc9PSIsInZhbHVlIjoiQmwxc2F3eElreGJ3bkNEMjVKWlMzc2ROYWk4ZnlicThVcWt5RExzZVFmTT0iLCJtYWMiOiI3MmQzYTY4MTk4N2ZlNDYyNjExOWFhZTZiODE0MzJkZGY1ZmUwZWFjZDBjZTE0NjYzNmJkYWI4NmUyZTY5OGU1IiwidGFnIjoiIn0=)