.
Since the first procedure performed by Sigwart at the Royal Brompton Hospital of London in 1994, alcohol septal ablation (ASA) has become a highly popular technique for the treatment of obstruction in hypertrophic cardiomyopathy (HCM).1 Percutaneous procedures have revolutionized the treatment of ischemic heart disease and arrhythmias and, more recently, there has been a great upsurge in their use in the treatment of structural heart disease and valve diseases.
In recent years, the number of ASA procedures performed is in the region of 5- to 10-fold higher than the number of myectomies. As Maron et al. report in a recent article,2 myectomy has largely been replaced by ASA in some European countries. This circumstance has led to the demise of myectomy programs in many centers. Myectomy continues to be the technique of choice in countries like the United States, perhaps the United Kingdom, and Italy. It may be that the characteristics of the public health systems of certain countries favor the development of percutaneous techniques and make it difficult for referral centers with surgical experience to continue their former activity.
There are differences in the procedures, the complications, and the results associated with the two options that should be considered when choosing the most appropriate treatment for a given patient. It is important that the decision-making be shared among experienced professionals in units specialized in cardiomyopathies, with integrated programs for the invasive treatment of obstruction in HCM.
EfficacyThe first question that needs to be addressed is whether the efficacy of ASA in reducing obstruction is comparable to that of myectomy.
In the surgical intervention, the cardiac surgeon resects between 8 and 10 grams of myocardial tissue from the basal anterior septum, whereas in ASA a similar amount of tissue is ablated at a somewhat more posterior site in the septum3 (Figure 1). Despite the injection of the echocardiographic contrast, which enables the correct identification of the target region, the ablated portion of the myocardium is usually irregular, and is frequently displaced toward the right side of the septal wall. The occurrence of left bundle branch block (LBBB) is the rule in myectomy, whereas ASA is accompanied by the development of right bundle branch block in over 80% of the cases. (Figure 2). The occurrence of LBBB could be a secondary effect, in this case beneficial, of surgical myectomy. The loss of synchrony in left ventricular contraction is one of the desired effects that led to pacemaker implantation in many patients with HCM during the nineties. Pacing continues to be a valid option in certain situations and should be taken into account in cases in which myectomy is indicated following failed ASA or vice versa. Patients in whom a prophylactic implantable cardioverter defibrillator (ICD) is indicated who present with obstruction can benefit from echocardiography-guided adjustments in the programming of pacemaker function, with a short atrioventricular interval to allow continuous right ventricular apical pacing.
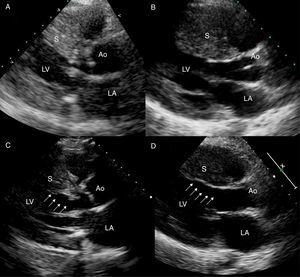
Figure 1. Reduction of septal thickness (arrows) and resolution of the obstruction. Echocardiogram at baseline (A) and 6 months after alcohol septal ablation (C) in a 67-year-old woman with a previous septal thickness of 24mm. Echocardiogram at baseline (B) and 1 month after myectomy (D) in a 20-year-old man with a previous septal thickness of 28mm. Ao, aorta; LA, left atrium; LV, left ventricle; S, septum.
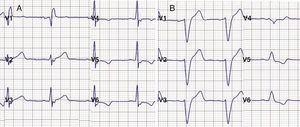
Figure 2. A: right bundle branch block after alcohol septal ablation. B: left bundle branch block after myectomy.
The reduction of the gradient is observed immediately after the intervention, whereas the benefits of ASA are delayed, sometimes for more than 6 months after alcohol injection. The average reduction in myocardial thickness produced by ASA is generally slight (approximately 5mm). Both myectomy and ASA are followed by a process of cardiac remodeling that involves the reduction of the thickness in other segments and in the size of the left atrium, a consequence of the hemodynamic improvement achieved. As indicated in the clinical practice guidelines,4 surgery is the treatment of choice in the presence of valve disease. Structural changes are often found in the mitral valve of patients with HCM, and it is sometimes difficult to discern to what extent the mitral regurgitation is due to anterior wall motion of the mitral valve or whether the problem is caused mainly by a structural valve abnormality. It is important to resort to all the available diagnostic tools prior to deciding which is the more appropriate procedure.
Despite the fact that there are no randomized studies comparing myectomy and ASA, a dozen studies, among them 2 recent meta-analyses, have reported similar results for the two procedures,5, 6 although the reduction of the gradient was slightly more marked with ASA. A study that compiles the short-term results and complications in 3000 ASA procedures documented in 42 reports was published in 20067; it shows a reduction in the resting and provoked gradients (from 65mmHg to 15mmHg and from 123mmHg to 35mmHg, respectively) and a significant improvement in the New York Heart Association functional class from 2.9 to 1.2. The improvements in functional class and exercise capacity following myectomy and following ASA were similar.
SafetyThe second question is related to safety: Are the two procedures associated with similar rates of acute and late complications?
In experienced centers, the periprocedural mortality is low, around 1%, with both myectomy and ASA. The most common complications of ASA are coronary artery dissection (∼1%), leakage of alcohol into a major coronary artery (<1%), and perforation of right ventricle or thrombosis associated with manipulation of the temporary pacemaker (∼2%), especially when it is necessary to maintain the device for more than 48hours. The complications associated with myectomy range from the development of atrial fibrillation to pericardial and pleural effusions that require the repositioning of drains (∼5%), infections, the development or persistence of mitral or aortic valve regurgitation, and the feared ventricular septal defect (∼1%).8, 9
Up to 50% of patients who undergo ASA develop complete atrioventricular block (AVB) in the catheterization laboratory or within 24hours of the procedure. In most of these cases, it is a self-limited phenomenon. Between 5% and 10% of the patients require implantation of a permanent pacemaker because the block persists. The incidence of permanent pacemaker implantation after ASA is significantly higher than that recorded after myectomy (odds ratio = 2.6).6
Patients with first-degree AVB and those with LBBB are at high risk for persistent advanced block during ASA; thus, the implantation of a permanent pacemaker prior to the procedure is recommended. One group of authors has analyzed a series of predictors of high-degree AVB; these include bradycardia, severe resting obstruction, persistence of AVB beyond the first 24hours, delay in reaching peak cardiac enzyme levels, and QRS duration.10 It is important to maintain monitoring for 1 week since late development of AVB has been reported in patients without conduction disturbances during the first hours after the intervention.
After the acute period and once the problem of blocks has been resolved, the most feared complication associated with ASA is the development of malignant ventricular arrhythmias. Whether this treatment is proarrhythmogenic remains a matter of debate. There are studies that demonstrate an association between the occurrence of late enhancement in cardiac magnetic resonance imaging and the development of nonsustained ventricular tachycardia.11, 12 Despite the fact that ablation produces a scar that can be detected and quantified by magnetic resonance, this relationship has not been clearly demonstrated in the context of ASA. In one of the earliest studies, in which the risk profiles of 100 consecutive patients were compared over a 1-year follow-up period, the authors observed a significant reduction in the risk markers for sudden death, such as syncope (31% vs 1%) or abnormal blood pressure response to stress testing (23% vs 1%), with no significant changes in nonsustained ventricular tachycardia (19% vs 19%).13
The beneficial impact of surgical myectomy on survival in patients with HCM has been clearly demonstrated. There are studies that show an improvement in overall survival following myectomy,8, 9 and there may be a reduction in the rate of sudden death. It has been argued that the hemodynamic improvement associated with the treatment would reduce not only events related to heart failure, but the risk of arrhythmias as well.
There is little information on the long-term complications of ASA compared to those of myectomy. The available data are provided by 2 recently published meta-analyses, one of which included 360 patients (5 studies)5 and the other 706 patients (12 studies).6 Neither of these meta-analyses revealed significant differences between the two therapeutic options in terms of mortality. Given the particularities of this disease, its marked heterogeneity, and its relatively low prevalence, it is highly improbable that the results of a randomized study that resolves some of these doubts will become available in the near future.14
Technical aspectsAs occurs with every technique after it is first described, the surgical treatment of obstruction underwent a development process in which different approaches were tested. In the sixties, there was debate as to whether the best treatment for obstruction was septal myotomy alone (in which a longitudinal incision was made in the septum without myocardial resection), myectomy with wedge resection of the septal wall, or isolated mitral valve replacement. On the other hand, with respect to the optimal approach, left ventricular apical, left ventricular, and transaortic access have all had their advocates. At the present time, the surgical technique is well accepted and is carried out with few differences between surgical teams from one hospital and country to another. Transaortic myectomy is now considered the technique of choice.
With regard to ASA, there are certain important aspects of the protocol that have yet to be established and that may be determinants of the acute and chronic results (Table 1). At most centers, the technique performed is that proposed by Faber et al.,15 which incorporates the assessment of the distribution of the echocardiographic contrast prior to alcohol injection. Some series that include patients treated during the early years of the development of the technique report a higher percentage of complications, a circumstance that could be related to the use of a greater volume of alcohol. In this respect, the results of one study published after the meta-analyses, which compares the results of ASA and myectomy in a center in The Netherlands,16 warrants special consideration. In that report, the annual rate of cardiovascular death or ICD discharge was 5.2-fold higher in the ASA group than in the myectomy group (4.4% vs 0.9%). Despite the fact that the study was not randomized, the characteristics of the patients in the 2 groups overlapped. The procedure was completed when resolution of the obstruction was achieved in the laboratory. The first patient of this series was included in 1999 and the first 25 patients received large volumes of alcohol (4.5mL), approximately twice the amount employed at the present time. Despite the fact that it is very common to observe a reduction in the gradient during the procedure, the beneficial effect is the consequence of a slow process of fibrosis and ventricular remodeling that is not achieved until some months later. The relationship between the acute results and the long-term benefits is poor. This report is highly illustrative of an entire series of technical aspects that remain to be defined, and could explain the differences from one center to another in terms of the results obtained. (Unfortunately, most of the studies on ASA do not describe the technique employed in such great detail.) The alcohol was administered in small boluses of 0.5mL separated by 5-min intervals during which saline was perfused. In the cases in which an acute reduction in the gradient was not achieved after the injection of 2.5mL of alcohol into a septal branch, a second branch was treated during the same procedure. Most groups postpone the treatment of a second branch, should it be necessary, for a few months, although there is no evidence that this is the best decision.
Table 1. Technical Aspects to Be Defined in the Alcohol Septal Ablation Procedure.
| Related to the septal branch | • Choice of main or segmental septal branch• Minimum size of the septal branch• Number of septal branches per procedure• Type of echocardiographic contrast |
| Alcohol injection | • Necessary volume of alcohol• Maximum volume of alcohol• Rate of injection• Occlusion time |
| Management of conduction disturbances | • Implantation of temporary pacemaker (prophylactic or demand device)• Time from onset of atrioventricular block to permanent pacemaker implantation• Risk markers for late atrioventricular block |
| Objectives and analysis of the results | • Immediate objectives (in the laboratory)• Time spent in the evaluation of the results• Risk markers for arrhythmia |
Thus, we find that we have an effective and safe percutaneous technique for the treatment of obstruction in HCM but that many highly important aspects of the procedure have yet to be defined.
Diagnostic and therapeutic tools under developmentTechnological advances have led to improvements in diagnostic techniques and have opened new treatment possibilities that enable us to better our knowledge of the pathophysiology of the obstruction, correctly identify candidates, and reduce the complications related to treatment.
From a diagnostic point of view, cardiac magnetic resonance imaging, computed tomography, transesophageal echocardiography, and 3-dimensional echocardiography are highly useful tools for determining whether invasive treatment is indicated and defining the strategy to be employed in the surgical option. Despite the fact that in most cases transthoracic echocardiography suffices for the guidance of ASA, transesophageal echocardiography may be necessary when the quality of the image is poor. With the development of interventional cardiology for the treatment of structural heart disease, new intravascular ultrasound probes have been introduced and may be helpful in this context, although to date the information on their use is merely anecdotal.17
Years ago, other variations of ASA using coils, spheres, and Teflon stents were tested but rapidly fell into disuse because of their lack of efficacy. More recently, the interesting results of a first study involving radiofrequency ablation applied to the septum from right ventricle or from left ventricle have been published.18 The reduction of the septal thickness in this study was marginal (1mm), despite multiple applications. The mechanism by which this technique produces a reduction of the left ventricular outflow tract gradient seems to be based on an effect combining hypokinesia and dyssynchrony due to branch block.
Plication of the anterior mitral valve leaflet and the Alfieri technique may be of use in some cases in which the anterior mitral leaflet is redundant and there is mild hypertrophy of the septal wall.19 Likewise, the mitral clip is an attractive technique for patients who are poor surgical candidates and in whom the major mechanism of the obstruction is anterior wall motion of the mitral valve, although one of the limitations of the clip in HCM is that its placement requires a ventricular cavity large enough for maneuvering.
The treatment of midventricular obstruction continues to be an unresolved problem; both ASA and surgery have been employed, with poorer results and a higher rate of complications compared to treating outflow tract obstruction. Some centers have published the results of small series of patients in which myocardial resection was performed through an apical incision.20 This technique could be an option in those cases of severe midapical concentric hypertrophy, with or without midventricular obstruction, in which the size of the ventricular cavity is minimal. It is important to consider the possibility of cardiac transplantation in these cases and in others in which the therapeutic options have been exhausted.
Finally, slight modifications in the technique contribute to the reduction of complications in ASA; these modifications include the use of new electrophysiology catheters with active fixation elements that reduce the risk of perforation related to the manipulation of the temporary pacemaker, or the use of jugular or subclavian venous access, facilitating patient mobility. Radial artery access offers the advantage that it reduces vascular complications, although the gauge of the guiding catheter is limited to 6 Fr. It is important to achieve an adequate contrast flow in order to maintain good visibility of the small-caliber septal branches, which usually requires 7-Fr catheters.
Access to invasive treatment of obstructionIt is very important that both therapeutic options be available, together with the specialists involved: clinical cardiologist, echocardiography specialist, interventionist, and cardiac surgeon. Moreover, close collaboration must be established with the anesthetists and intensivists responsible for the management of the patient during the intervention and postoperative period. The best alternatives for each patient should be discussed by the team and the decision made on an individual basis. Therapeutic guidelines and expert consensus documents recommend either of the options in cases of dyspnea (New York Heart Association functional class ≥ III) in relation to the obstruction (>50mmHg, resting, or provoked). Surgery is the treatment of choice when there is concomitant structural valve disease. Given the features of the two options, although this aspect is not mentioned in the guidelines, surgery appears to be more suitable in young patients or in those with severe hypertrophy (>25mm). Older patients with less severe disease and those who have undergone pacemaker or ICD implantation, if their anatomy is favorable, could be good candidates for ASA.
The introduction of myectomy and ASA programs differs from one country or hospital to another. Given that the percutaneous technique is relatively easier to perform, ASA programs have grown exponentially, to the detriment of surgery. It is presently uncommon to find both therapeutic options performed by professionals with the same degree of experience in the same center, or even in the same country. In some European countries, myectomy is seldom performed, or simply is not offered, at centers with cardiomyopathy units. On the other hand, centers with a long tradition of myectomy, such as large surgical centers in the United States, account for the majority of the percutaneous interventions that are currently being done in the entire world. One advantage of the percutaneous technique is that it can be performed in all the hospitals in which interventional procedures are carried out; in contrast, the disadvantages are that it is performed in centers in which there is limited experience and that the cases are too widely dispersed.
In conclusion, to ensure the success of invasive treatment, it is important that an experienced multidisciplinary team determine with absolute certainty whether or not it is indicated and choose the correct approach, whether surgical or percutaneous. Alcohol septal ablation has become a valid alternative to myectomy for most patients, although it is necessary to make an effort to establish a clear, consensus-based protocol. In this respect, we need to promote the creation of prospective, multicenter registries that enable us to confirm the long-term safety of this technique.
Conflicts of interestNone declared.
Corresponding author: The Heart Hospital, 16-18 Westmoreland St., London W1 8PH, United Kingdom. william.mckenna@uclh.org