There is little evidence on rates of stent thrombosis (ST) in patients receiving dual antiplatelet therapy (DAPT) with ticagrelor or prasugrel. The aim of this study was to analyze the incidence and predictors of ST after an acute coronary syndrome among patients receiving DAPT with ticagrelor vs prasugrel.
MethodsWe used data from the RENAMI registry (REgistry of New Antiplatelet therapy in patients with acute Myocardial Infarction), analyzing a total of 4123 acute coronary syndrome patients discharged with DAPT with ticagrelor or prasugrel in 11 centers in 6 European countries. The endpoint was definite ST within the first year. A competitive risk analysis was carried out using a Fine and Gray regression model, with death being the competitive event.
ResultsA total of 2604 patients received DAPT with ticagrelor and 1519 with prasugrel; ST occurred in 41 patients (1.10%), with a similar cumulative incidence between ticagrelor (1.21%) and prasugrel (0.90%). The independent predictors of ST were age (sHR, 1.03; 95%CI, 1.01-1.06), ST segment elevation (sHR, 2.24; 95%CI, 1.22-4.14), previous myocardial infarction (sHR, 2.56; 95%CI, 1.19-5.49), and serum creatinine (sHR, 1.29; 95%CI, 1.08-1.54).
ConclusionsStent thrombosis is infrequent in patients receiving DAPT with ticagrelor or prasugrel. The variables associated with an increased risk of ST were advanced age, ST segment elevation, previous myocardial infarction, and serum creatinine.
Keywords
Because patients with acute coronary syndrome (ACS) have excessive platelet reactivity, antiplatelet therapy is vital to prevent new ischemic events.1 New antiplatelet agents such as prasugrel and ticagrelor have been developed in recent years. Both drugs show greater ability than clopidogrel to reduce stent thrombosis (ST).2,3 However, the available data on the impact of prasugrel and ticagrelor on ST events are mainly derived from clinical trials, and little patient information is available from clinical practice. In addition, few studies have compared the therapeutic benefit, in terms of ST rates, between prasugrel and ticagrelor.4–7
The aim of this study was to evaluate, from a descriptive-analytical point of view, the incidence and predictors of ST in patients with ACS receiving dual antiplatelet therapy (DAPT) with aspirin plus prasugrel or ticagrelor using data from an international registry of patients undergoing percutaneous coronary revascularization.
METHODSStudy PopulationThe RENAMI (REgistry of New Antiplatelet therapy in patients with Myocardial Infarction) is a retrospective, observational, multicenter, and international registry involving the voluntary participation of 11 centers from 6 European countries (Spain, Italy, Switzerland, Greece, Serbia, and the United Kingdom). The objective of this unfunded registry is to improve our understanding of ischemic and hemorrhagic complications in patients with ACS treated with DAPT with prasugrel or ticagrelor. All participating centers are third-level university hospitals with a 24-hour catheterization laboratory and internal medical records on ACS. From January 2012 to January 2016, each participating center consecutively enrolled patients with ACS and coronary stenosis ≥ 50% in the left main coronary artery and ≥ 70% in the rest of the coronary tree who underwent percutaneous coronary intervention (PCI). Additionally, these patients were discharged with DAPT comprising aspirin (100mg/24h) plus prasugrel (10mg/24h) or aspirin (100mg/24h) plus ticagrelor (90mg/12h). The design of the RENAMI registry and the inclusion and exclusion criteria are described in detail in the supplementary material. The variables included in the RENAMI registry are listed in and the participating centers in . The ACS was defined as ST-segment elevation myocardial infarction (STEMI), non-STEMI, and unstable angina according to the definitions accepted in clinical practice guidelines.8–10 Acute myocardial infarction (AMI) was diagnosed according to the universal definition of AMI.11 Unstable angina was diagnosed through the presence of its classic symptoms or objective evidence of myocardial ischemia in a stress test, together with detection of a culprit lesion on coronary angiography.
For the purpose of RENAMI, a database was designed to retrospectively include information on clinical, analytical, and angiographic variables, as well as data related to mortality and ischemic and hemorrhagic events. RENAMI registry analyses were carried out by 2 researchers from the coordination center (E. Abu-Assi and S. Raposeiras-Roubín). All procedures were performed according to the provisions of the Declaration of Helsinki and with the approval of the local ethics committees.
Of the 4424 patients in the RENAMI registry, the present study excluded those with no information on the type of stent implanted (n=297) and those with a first-generation drug-eluting stent (DES) (n=4). Thus, the final study cohort comprised 4123 patients.
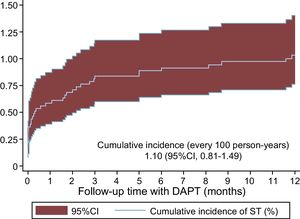
Endpoint, Definitions, and Follow-upThe study endpoint was ST in patients receiving DAPT with ticagrelor or prasugrel, considering only thrombosis confirmed according to the definition of the Academic Research Consortium (angiographic or pathologic confirmation).12 STs were classified as early (occurring in the first month after PCI) or late (occurring between 1 and 12 months after PCI). In addition, early STs were subdivided into acute (first 24hours) and subacute (between 1 and 30 days). Stent thromboses occurring in the acute phase before the administration of prasugrel or ticagrelor (in the case of patients initially treated with DAPT with clopidogrel) were excluded from the event file because the objective was to study the ST rates of patients on DAPT with ticagrelor or prasugrel. Follow-up was censored at ST occurrence (n=41), death (n=72), DAPT suspension/withdrawal within 1 year after the PCI (n=382), or at 12 months of follow-up.
Analysis of the composite endpoint of death and/or ST is shown in . This secondary analysis was performed due to the small number of ST events.
Data on vital status (alive or dead) and event (ST) were obtained from hospital clinical data records and administrative records (vital statistics records, hospital discharge data, and emergency department data). When information was missing, patients or their relatives or primary care physicians were contacted by telephone.
According to a report of the European Society of Cardiology and the European Association of PCI,13 all stents succeeding the first-generation stents were considered new-generation DESs; the first-generation stents were the sirolimus-eluting CYPHER, paclitaxel-eluting TAXUS, and zotarolimus-eluting Endeavor. Thus, “new-generation DESs” refer to the everolimus-eluting XIENCE V (Abbott Vascular, Illinois, United States) and Promus (Boston-Scientific, Massachusetts, United States), zotarolimus-eluting Resolute (Medtronic; Minnesota, United States), sirolimus-eluting Yukon DES (Translumina GmbH; Hechingen, Germany), and biolimus-eluting Nobori (Terumo, Japan) and Biomatrix (Biosensors, Singapore). Bioabsorbable stents were also considered new-generation DESs.
Statistical AnalysisContinuous variables are presented as mean±standard deviation. Discrete variables are expressed as percentages. Continuous variables were compared with the Student t test and discrete variables with the chi-square test or Fisher exact test, as necessary.
To study ST predictors, a competing risk analysis was performed with a Fine and Gray regression model,14 with death considered the competing event. All variables associated (P <.10) with ST in univariable analysis were included in a multivariable model. There was no evidence of noncompliance with the proportional hazards assumption, verified by studying the interaction of covariables in the models with time: in the absence of statistical significance (P> .05), it can be deduced that the proportional hazards assumption has not been violated. To account for the potential heterogeneity arising from the inclusion of centers from different European countries, an interaction term was included in the final model through a nonhierarchical grouping analysis by group (country). The predictive accuracy of the final model was calculated via the C-statistic, using the c-index function of the “pec” extension for R. Results are expressed as the subhazard ratio (sHR) with its corresponding 95% confidence interval (95%CI).
Cumulative incidence function curves were plotted for ticagrelor and prasugrel after estimation of the cumulative incidence function curve using a flexible parametric model for competing risks,15 adjusted for the variables associated (P <.10) in the univariable analysis with a higher incidence of ST (Table 1): age, previous myocardial infarction, STEMI presentation, left ventricular ejection fraction <40%, and serum creatinine.
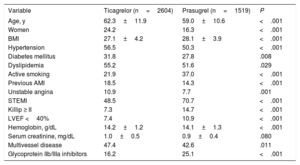
Baseline Characteristics of the Study Population According to Whether the Dual Antiplatelet Therapy Involved Ticagrelor or Prasugrel
| Variable | Ticagrelor (n=2604) | Prasugrel (n=1519) | P |
|---|---|---|---|
| Age, y | 62.3±11.9 | 59.0±10.6 | <.001 |
| Women | 24.2 | 16.3 | <.001 |
| BMI | 27.1±4.2 | 28.1±3.9 | <.001 |
| Hypertension | 56.5 | 50.3 | <.001 |
| Diabetes mellitus | 31.8 | 27.8 | .008 |
| Dyslipidemia | 55.2 | 51.6 | .029 |
| Active smoking | 21.9 | 37.0 | <.001 |
| Previous AMI | 18.5 | 14.3 | <.001 |
| Unstable angina | 10.9 | 7.7 | .001 |
| STEMI | 48.5 | 70.7 | <.001 |
| Killip ≥ II | 7.3 | 14.7 | <.001 |
| LVEF <40% | 7.4 | 10.9 | <.001 |
| Hemoglobin, g/dL | 14.2±1.2 | 14.1±1.3 | <.001 |
| Serum creatinine, mg/dL | 1.0±0.5 | 0.9±0.4 | .080 |
| Multivessel disease | 47.4 | 42.6 | .011 |
| Glycoprotein IIb/IIIa inhibitors | 16.2 | 25.1 | <.001 |
AMI, acute myocardial infarction; BMI, body mass index; LVEF, left ventricular ejection fraction; STEMI, ST-segment elevation acute myocardial infarction.
Values are expressed as percentage or mean±standard deviation.
The composite analysis of death and ST () was performed using a Cox model; ticagrelor and prasugrel curves were compared by a log-rank test and the restricted mean survival time method.16
Statistical analyses were performed with Stata 13.1 and R version 3.3.1. P <.05 was considered statistically significant.
RESULTSStudy Population CharacteristicsThe clinical characteristics of the study population are shown in Table 1 according to use of ticagrelor and prasugrel. The 2 drugs clearly display different prescription patterns.
Regarding DAPT, 63.2% of patients (n=2604) received ticagrelor and 36.8% (n=1519) received prasugrel. The mean follow-up time was 10.9±2.9 months; 82.9% of patients completed 12 months of follow-up with DAPT (94.4%> 6 months, 88.4%> 9 months).
Bare-metal stents were used in 29.2% of the patients (n=1205) and new-generation DESs in the remaining 70.8% (n=2918). Only 4.0% of patients treated with DESs received bioabsorbable stents (n=117): 24.8% received zotarolimus-eluting stents; 24.4%, everolimus-eluting; 12.8%, biolimus-eluting; and 3.1%, sirolimus-eluting. The remaining 30.9% received new-generation DESs (nonbioabsorbable) but the type was not specified.
Stent Thrombosis IncidenceOf the 4123 patients with ACS treated with DAPT with ticagrelor and/or prasugrel, 41 experienced ST in the first year (1.10/100 person-years, 95%CI, 0.81-1.49) (Figure 1); 58.6% of the STs were early, occurring in the first month (36.6% in the first 24h and 22.0% between the first day and the first month), whereas 41.5% were late, occurring between the first month and the first year.
Regarding stent type, there were no significant differences in the cumulative incidences of ST between bare-metal stents (0.83%; 95%CI, 0.43-1.60) and new-generation DESs (1.20%; 95%CI, 0.85-1.70). Bioabsorbable stents were implanted in 127 patients; of these, only 1 experienced a ST in the first year.
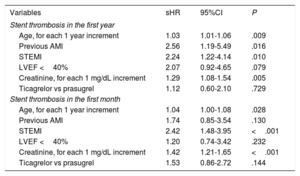
Stent Thrombosis PredictorsThe association between the clinical variables analyzed and a higher incidence of ST in the first year in the univariable analysis is shown in Table 2. Multivariable analysis identified the following independent predictors of ST in the first year: age, STEMI presentation, previous AMI, and serum creatinine (Table 3). The discriminative ability of the final model was good, with a C-statistic of 0.70. The same variables were significant in the univariable analysis if it was limited to ST in the first month (Table 2). However, after multivariable adjustment, the only independent predictors of early ST were age, STEMI presentation, and serum creatinine (Table 3).
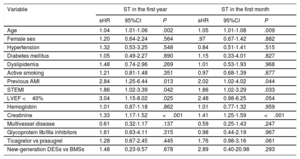
Univariable Analysis of Stent Thrombosis in the First Year and in the First Month
| Variable | ST in the first year | ST in the first month | ||||
|---|---|---|---|---|---|---|
| sHR | 95%CI | P | sHR | 95%CI | P | |
| Age | 1.04 | 1.01-1.06 | .002 | 1.05 | 1.01-1.08 | .009 |
| Female sex | 1.20 | 0.64-2.24 | .564 | .97 | 0.67-1.42 | .882 |
| Hypertension | 1.32 | 0.53-3.25 | .548 | 0.84 | 0.51-1.41 | .515 |
| Diabetes mellitus | 1.05 | 0.49-2.27 | .890 | 1.15 | 0.33-4.01 | .827 |
| Dyslipidemia | 1.48 | 0.74-2.96 | .269 | 1.01 | 0.53-1.93 | .968 |
| Active smoking | 1.21 | 0.81-1.48 | .351 | 0.97 | 0.68-1.39 | .877 |
| Previous AMI | 2.84 | 1.25-6.44 | .013 | 2.02 | 1.02-4.02 | .044 |
| STEMI | 1.86 | 1.02-3.39 | .042 | 1.86 | 1.02-3.29 | .033 |
| LVEF <40% | 3.04 | 1.15-8.02 | .025 | 2.48 | 0.98-6.25 | .054 |
| Hemoglobin | 1.01 | 0.87-1.18 | .862 | 1.01 | 0.77-1.32 | .959 |
| Creatinine | 1.33 | 1.17-1.52 | <.001 | 1.41 | 1.25-1.59 | <.001 |
| Multivessel disease | 0.61 | 0.32-1.17 | .137 | 0.59 | 0.25-1.43 | .247 |
| Glycoprotein IIb/IIIa inhibitors | 1.61 | 0.63-4.11 | .315 | 0.98 | 0.44-2.19 | .967 |
| Ticagrelor vs prasugrel | 1.28 | 0.67-2.45 | .445 | 1.76 | 0.98-3.16 | .061 |
| New-generation DESs vs BMSs | 1.48 | 0.23-9.57 | .678 | 2.89 | 0.40-20.98 | .293 |
95%CI, 95% confidence interval; AMI, acute myocardial infarction; BMS, bare-metal stent; DES, drug-eluting stent; LVEF, left ventricular ejection fraction; sHR, subhazard ratio; STEMI, ST-segment elevation acute myocardial infarction; ST, stent thrombosis.
Multivariable Analysis for Predicting Stent Thrombosis in the First Year and Particularly in the First Month
| Variables | sHR | 95%CI | P |
|---|---|---|---|
| Stent thrombosis in the first year | |||
| Age, for each 1 year increment | 1.03 | 1.01-1.06 | .009 |
| Previous AMI | 2.56 | 1.19-5.49 | .016 |
| STEMI | 2.24 | 1.22-4.14 | .010 |
| LVEF <40% | 2.07 | 0.92-4.65 | .079 |
| Creatinine, for each 1 mg/dL increment | 1.29 | 1.08-1.54 | .005 |
| Ticagrelor vs prasugrel | 1.12 | 0.60-2.10 | .729 |
| Stent thrombosis in the first month | |||
| Age, for each 1 year increment | 1.04 | 1.00-1.08 | .028 |
| Previous AMI | 1.74 | 0.85-3.54 | .130 |
| STEMI | 2.42 | 1.48-3.95 | <.001 |
| LVEF <40% | 1.20 | 0.74-3.42 | .232 |
| Creatinine, for each 1 mg/dL increment | 1.42 | 1.21-1.65 | <.001 |
| Ticagrelor vs prasugrel | 1.53 | 0.86-2.72 | .144 |
95%CI, 95% confidence interval; AMI, acute myocardial infarction; LVEF, left ventricular ejection fraction; sHR, subhazard ratio; STEMI, ST-segment elevation myocardial infarction.
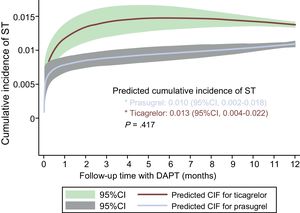
The cumulative incidence of ST occurring in the first year was 1.21% (95%CI, 0.53-1.56) with ticagrelor and 0.90% (95%CI, 0.84-1.76) with prasugrel. There were no significant differences between the 2 drugs regarding the risk of ST in the first year (ticagrelor vs prasugrel, sHR=1.29; 95%CI, 0.67-2.45; P=.445). The adjusted curves of the cumulative incidence of ST estimated with ticagrelor and prasugrel in the first year are shown in Figure 2. There were also no significant differences between ticagrelor and prasugrel in terms of ST in the first month (0.7% vs 0.4%; P=.144).
Cumulative incidence of ST estimated according to the type of antiplatelet agent, after adjustment for age, previous myocardial infarction, presentation as ST-segment elevation acute myocardial infarction, left ventricular ejection fraction <40%, and serum creatinine. 95%CI, 95% confidence interval; CIF, cumulative incidence function curve; DAPT, dual antiplatelet therapy; ST, stent thrombosis.
No associations were found for ST risk with ticagrelor vs prasugrel, bare-metal stents (ticagrelor vs prasugrel, sHR=1.40; 95%CI, 0.35-5.56; P=.635), or new-generation DESs (sHR=1.23; 95%CI, 0.58-2.58; P=.587).
DISCUSSIONThe most interesting findings of this study can be summarized in 4 points: a) the incidence of ST in patients with ACS on DAPT with ticagrelor or prasugrel is low, and the risk is highest in the first month; b) age, previous infarction, STEMI presentation, and renal function are the main predictors of ST in patients treated with ticagrelor or prasugrel; c) in patients who received DAPT with ticagrelor or prasugrel, no differences in ST risk were found between bare-metal stents and new-generation DESs; and d) in general, there was no difference between prasugrel and ticagrelor regarding the risk of ST in the first year, although higher-powered studies are needed to accurately compare the 2 drugs in this regard.
The annual incidence of ST is highly variable and highly dependent on the interaction of different factors, such as the study population (with or without ACS), type of antiplatelet agent used (clopidogrel or the new P2Y12 inhibitors), and type of stent (bare-metal stents, first-generation DESs, new-generation DESs, bioabsorbable DESs).17–19 Thus, the first-year ST incidence in the literature ranges from 0.5% to 1.5%, with between 50% and 70% occurring in the first month,20,21 data that are similar to ours. The present study focuses on a contemporary population of patients with ACS (more than 50% with STEMI), all treated with DAPT with ticagrelor (63.2%) or prasugrel (36.8%) and implanted with either a bare-metal stent (29.2%) or new-generation DES (70.8%). In this population context, the incidences of ST are reported in patients with ongoing DAPT (because the termination of this therapy was a motive for censoring of follow-up, as explained in the “Methods”), which is one of the strengths of this study. This is particularly interesting because it presents an ideal scenario to mitigate ST: treatment with stents other than first-generation DESs and DAPT with new and more potent inhibitors of P2Y12. This population group showed a cumulative incidence of ST in the first year of 1.1% (0.6% in the first month), despite comprising patients on DAPT with ticagrelor or prasugrel. This rate reflects the complex pathophysiology of ST due to the involvement of multiple interrelated factors, in addition to the DAPT itself. Indeed, in the PESTO (Morphological Parameters Explaining Stent Thrombosis) registry, a mechanical abnormality of the implanted stent was observed on optical coherence tomography in 97% of patients with ST.22 Recently, a study by Cuesta et al.23 also showed through optical coherence tomography that the predominant mechanism in 39% of early ST was stent malapposition.
Independent predictors of ST in patients treated with DAPT with ticagrelor or prasugrel were persistent ST-segment elevation, previous AMI, and renal failure. These factors were also identified in other studies and reflect situations of greater thrombogenicity (STEMI, recurrent AMI, ventricular dysfunction) and coagulopathy (age, renal failure).24–27
Both ticagrelor and prasugrel were associated with a marked reduction in ST vs clopidogrel in the pivotal clinical trials. The PLATO study showed a 33% reduction in the 1-year incidence of definite ST with ticagrelor vs clopidogrel (1.93% vs 1.37%, hazard ratio [HR]=0.67; 95%CI, 0.50-0.90; P=.009),2 which was later confirmed in the SWEDEHEART registry.28 In the TRITON study, the 15-month incidence of definite ST was 2.2% in the clopidogrel group but 1.1% in the prasugrel group.3 Regarding the “real life” population, that is, beyond patients included in clinical trials, only indirect data have been available from a limited number of registries.4–7 In our study, no significant differences were found between the 2 drugs (ticagrelor and prasugrel) in the cumulative incidence of ST at 1 year (1.2% with ticagrelor, 0.9% with prasugrel) or at 30 days (0.7% with ticagrelor, 0.4% with prasugrel). Although there has been little evidence of the true incidence of ST with prasugrel and ticagrelor in the setting of an ACS, the data obtained agree with those published in the most recent studies. In the PRAGUE study,6 in the setting of STEMI, the 1-month incidences of definite ST were 0.9% with ticagrelor and 0.5% with prasugrel; this difference was also not statistically significant but was limited by the small sample size (634 patients with prasugrel and 596 with ticagrelor). Previously, Larmore et al.5 published data from an American registry of patients with ACS with a considerable sample size (13 134 patients with prasugrel and 2964 with ticagrelor). The authors observed 30-day ST rates of 0.7% with ticagrelor and 0.3% with prasugrel, with a significant association in favor of prasugrel in the univariable analysis, although the benefit was diluted after propensity score matching (relative risk=0.56; 95%CI, 0.26-1.20; P=.131). Therefore, given the concordance of the data among the different studies, it seems reasonable to ask whether the absence of a significant difference between ticagrelor and prasugrel for ST is due to a power problem in the different studies (in order for a difference such as that obtained to be significant with an ST incidence similar to the one reported here, the sample size would have to exceed 14 000 patients with 95% CI and 80% power) or differences in baseline characteristics between patients treated with ticagrelor and prasugrel. This supposition leads to an interesting working hypothesis for future studies, with a view to determining whether prasugrel vs ticagrelor actually reduces the risk of ST, especially that of early ST. Although this greatly depends on procedural variables, residual platelet reactivity despite treatment has been associated with a marked increase in the risk of early ST. In fact, the factor that is alone associated with an increased risk of early ST is absence of platelet P2Y12 receptor inhibition.29 However, because the comparisons between the 2 drugs in terms of platelet inhibition were similar, and even better with ticagrelor,30–33 the results are difficult to understand from a pharmacodynamic point of view.
Regarding stent type, no differences were found in this study between ticagrelor and prasugrel in the ST rates of patients treated with bare-metal stents or new-generation DESs. Current evidence comparing the 2 types of stent shows equivalent results or even favorable results for new-generation DESs in terms of thrombogenicity.34,35 However, the findings are based on a population that generally received DAPT with clopidogrel, whose platelet inhibition is slower and more variable than that of ticagrelor and prasugrel. The more biocompatible polymer coatings of the new DESs have been suggested to reduce the thrombogenicity associated with the interaction between the stent and the blood,36 but little is known about the interaction between the type of stent and the type of antiplatelet agent. Thus, this study provides new information by revealing the ST rates in patients on DAPT with ticagrelor or prasugrel according to type of stent (bare-metal or new-generation DES). No significant differences were found in our registry between bare-metal stents and new-generation DESs, although the study was not designed for that purpose.
LimitationsThe 2 main limitations of this study lie in its retrospective design and in the sample size. The study suffers from the same limitations as other retrospective studies. Specifically, because it is not randomized and ticagrelor and prasugrel use are at the discretion of the treating physician, comparisons between these drugs or between bare-metal stents and DESs should be interpreted with caution due to the possible interaction of unmeasured confounding variables. For example, RENAMI does not provide information on angiographic characteristics possibly critically involved, particularly in early ST, such as TIMI (Thrombolysis In Myocardial Infarction) flow after PCI, stent diameter/length, residual untreated coronary dissection, and high residual thrombotic load. There is also no information on how many patients underwent intracoronary imaging after PCI to verify proper stent expansion. In addition, the sample size of the study, with 4123 patients, is insufficient to reach robust conclusions due to the low frequency of the study event (ie, ST), as already mentioned. However, given the high lethality posed by ST and the limited scientific evidence available on this event in patients on DAPT with ticagrelor or prasugrel, the present study—even with a more descriptive than analytical approach—has scientific value in hypothesis generation for subsequent scientific studies (clinical trials or meta-analyses).
CONCLUSIONSST was infrequent in patients on DAPT with ticagrelor or prasugrel. Four independent predictors were associated with higher ST risk: previous infarction, STEMI presentation, age, and serum creatinine. Antiplatelet agent and stent type were not associated with an increased risk of ST in the present study. Designs with higher power are required for comparative studies of the ability of prasugrel and ticagrelor to protect against ST.
CONFLICTS OF INTERESTE. Abu-Assi is an Associate Editor of Revista Española de Cardiología.
- –
Stent thrombosis is a dreaded complication after percutaneous coronary revascularization due to its high lethality. However, its incidence is currently low with the use of bare-metal stents and new-generation DESs. In addition, a reduced ST rate has been found with both ticagrelor and prasugrel vs clopidogrel.
- –
Beyond the pivotal clinical trials (PLATO and TRITON-TIMI), little is known about the incidence and predictors of ST in patients receiving DAPT with ticagrelor or prasugrel. This study analyzes both the incidence and predictors of ST after ACS in patients on DAPT with aspirin plus ticagrelor or aspirin plus prasugrel after percutaneous revascularization with bare-metal stents or new-generation DESs.