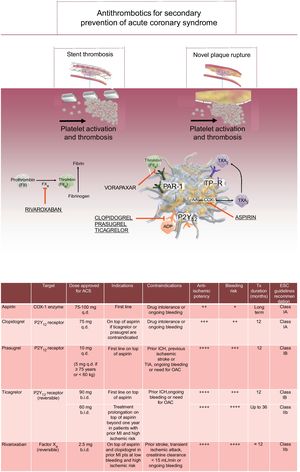
Antithrombotic treatment after an acute coronary syndrome (ACS) plays a key role in reducing adverse events, but poses serious difficulties owing to the delicate equilibrium between ischemic and bleeding risk.1 Patients with ACS are by definition at high ischemic risk, as their phenotype entails a tendency toward plaque rupture and vascular thrombosis.2–4 However, most also carry comorbidities that expose them to an excessive risk of bleeding during antithrombotic therapy, which can equally impact prognosis.5 For this reason, treatment adjustment based on the patient's characteristics is advocated by international guidelines.6 It is of the upmost importance to define clear options for patients with ACS, which include both the type and duration of dual antiplatelet therapy (DAPT).
TYPE OF DUAL ANTIPLATELET THERAPY AFTER AN ACUTE CORONARY SYNDROMEDAPT is the treatment of choice for secondary prevention of ACS.6 This is based on dual inhibition of platelet activation, with aspirin acting through the cyclooxygenase-1 pathway, and clopidogrel, prasugrel or ticagrelor blocking the P2Y12 receptor (Figure). While clopidogrel represented for many years the treatment of choice for secondary prevention of ACS, in the last 10 years, 2 landmark studies demonstrated the superiority of prasugrel and ticagrelor in this setting.3,4 Current international guidelines advocate the use of prasugrel or ticagrelor as first-line treatment in patients with ACS, while clopidogrel is restricted to those with a contraindication to potent antiplatelet agents, as is the case of those needing long-term oral anticoagulation.6
Oral antithrombotic drugs approved for secondary prevention after an acute coronary syndrome: molecular targets, mechanisms of action and clinical indications/contraindications for oral antithrombotic drugs approved in ACS. ACS, acute coronary syndrome; ADP, adenosine diphosphate; b.i.d., twice daily; COX-1, cyclooxygenase 1; ESC, European Society of Cardiology; FII, factor II; FX, factor X; ICH, intracranial hemorrhage; MI, myocardial infarction; OAC, oral anticoagulants; P2Y12, P2Y12 platelet receptors; PAR-1, protease activated receptor; q.d., once daily; TIA, transient ischemic attack; TP-R, thromboxane receptor; Tx, therapy; TXA2, thromboxane A2.
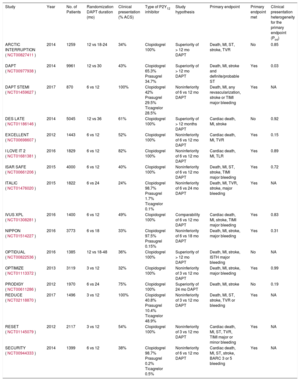
The recommended duration of DAPT in patients with ACS is generally set at 12 months by most international guidelines. This time period has been arbitrarily selected following the performance of the CURE trial, the first clinical trial testing DAPT with aspirin and clopidogrel in ACS patients.2 Nevertheless, the optimal DAPT duration has been a fervent topic of clinical research, with 16 randomized controlled trials testing different DAPT duration strategies that challenged the recommended standard of 12 months (Table). These studies can be grouped by 2 different hypotheses: studies testing the noninferiority of reducing DAPT duration to 3 or 6 months, and studies testing the superiority of extending DAPT duration beyond 12 months. In summary, it emerged that a longer treatment with DAPT is associated with a significant benefit in terms of recurrent stent or nonstent related ischemic events, but also with a significant increase in bleeding. In contrast, shorter DAPT significantly reduced the risk of bleeding among the spectrum of DAPT duration, while the impact on mortality or cardiovascular mortality remains controversial. Understanding which strategy carries the best net clinical benefit, in terms of the absolute rate of both ischemia and bleeding, is of paramount importance for optimal decision-making. Importantly, it has been demonstrated that several factors, such as clinical presentation and anatomical complexity,7,8 may play a role in the selection of treatment duration. Hence, treatment individualization may aid the selection of the optimal strategy.9
Randomized Controlled Trials Testing Dual Antiplatelet Therapy Duration Strategies Among Patients With an Acute Coronary Syndrome
| Study | Year | No. of Patients | Randomization DAPT duration (mo) | Clinical presentation (% ACS) | Type of P2Y12 inhibitor | Study hypothesis | Primary endpoint | Primary endpoint met | Clinical presentation heterogeneity for the primary endpoint (Pint) |
|---|---|---|---|---|---|---|---|---|---|
| ARCTIC INTERRUPTION (NCT00827411) | 2014 | 1259 | 12 vs 18-24 | 34% | Clopidogrel 100% | Superiority of > 12 mo DAPT | Death, MI, ST, stroke, TVR | No | 0.85 |
| DAPT (NCT00977938) | 2014 | 9961 | 12 vs 30 | 43% | Clopidogrel 65.3% Prasugrel 34.7% | Superiority of > 12 mo DAPT | Death, MI, stroke and definite/probable ST | Yes | 0.03 |
| DAPT STEMI (NCT01459627) | 2017 | 870 | 6 vs 12 | 100% | Clopidogrel 42% Prasugrel 29.5% Ticagrelor 28.5% | Noninferiority of 6 vs 12 mo DAPT | Death, MI, any revascularization, stroke or TIMI major bleeding | Yes | NA |
| DES LATE (NCT01186146) | 2014 | 5045 | 12 vs 36 | 61% | Clopidogrel 100% | Superiority of > 12 months DAPT | Cardiac death, MI, stroke | No | 0.92 |
| EXCELLENT (NCT00698607) | 2012 | 1443 | 6 vs 12 | 52% | Clopidogrel 100% | Noninferiority of 6 vs 12 mo DAPT | Cardiac death, MI, TVR | Yes | 0.15 |
| I LOVE IT 2 (NCT01681381) | 2016 | 1829 | 6 vs 12 | 82% | Clopidogrel 100% | Noninferiority of 6 vs 12 mo DAPT | Cardiac death, MI, TLR | Yes | 0.89 |
| ISAR SAFE (NCT00661206) | 2015 | 4000 | 6 vs 12 | 40% | Clopidogrel 100% | Noninferiority of 6 vs 12 mo DAPT | Death, MI, ST, stroke, TIMI major bleeding | Yes | 0.72 |
| ITALIC (NCT01476020) | 2015 | 1822 | 6 vs 24 | 24% | Clopidogrel 98.7% Prasugrel 1.7% Ticagrelor 0.1% | Noninferiority of 6 vs 24 mo DAPT | Death, MI, TVR, stroke, major bleeding | Yes | NA |
| IVUS XPL (NCT01308281) | 2016 | 1400 | 6 vs 12 | 49% | Clopidogrel 100% | Comparability of 6 vs 12 mo DAPT | Cardiac death, MI, stroke, TIMI major bleeding | Yes | 0.83 |
| NIPPON (NCT01514227) | 2016 | 3773 | 6 vs 18 | 33% | Clopidogrel 97.5% Prasugrel 0.15% | Noninferiority of 6 vs 18 mo DAPT | Death, MI, stroke, major bleeding | Yes | 0.31 |
| OPTIDUAL (NCT00822536) | 2016 | 1385 | 12 vs 18-48 | 36% | Clopidogrel 100% | Superiority of > 12 mo DAPT | Death, MI, stroke, ISTH major bleeding | No | NA |
| OPTIMIZE (NCT01113372) | 2013 | 3119 | 3 vs 12 | 32% | Clopidogrel 100% | Noninferiority of 3 vs 12 mo DAPT | Death, MI, stroke, major bleeding | Yes | 0.99 |
| PRODIGY (NCT00611286) | 2012 | 1970 | 6 vs 24 | 75% | Clopidogrel 100% | Superiority of 24 mo DAPT | Death, MI, stroke | No | 0.19 |
| REDUCE (NCT02118870) | 2017 | 1496 | 3 vs 12 | 100% | Clopidogrel 40.8% Prasugrel 10.4% Ticagrelor 48.9% | Noninferiority of 3 vs 12 mo DAPT | Death, MI, ST, stroke, TVR or bleeding | Yes | NA |
| RESET (NCT01145079) | 2012 | 2117 | 3 vs 12 | 54% | Clopidogrel 100% | Noninferiority of 3 vs 12 mo DAPT | Cardiac death, MI, ST, TVR, TIMI major or minor bleeding | Yes | NA |
| SECURITY (NCT00944333) | 2014 | 1399 | 6 vs 12 | 38% | Clopidogrel 98.7% Prasugrel 0.2% Ticagrelor 0.5% | Noninferiority of 6 vs 12 mo DAPT | Cardiac death, MI, ST, stroke, BARC 3 or 5 bleeding | Yes | NA |
ACS, acute coronary syndrome; BARC, Bleeding Academic Research Consortium; DAPT, dual antiplatelet therapy; ISTH, International Society of Thrombosis and Haemostasis; NA, not available; MI, myocardial infarction; ST, stent thrombosis; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombosis in myocardial infarction; TVR, target vessel revascularization.
Dedicated risk scores to calculate individual patient risk for adverse events have been recently introduced to inform decision-making for DAPT duration, and have been endorsed by international guidelines.6 The PRECISE-DAPT score, which includes 5 clinical risk factors to gauge individual patient risk of bleeding at the time of stent implantation, has been developed from a pooled dataset of 8 randomized controlled trials and a total 14 963 patients with an indication for DAPT who underwent elective, urgent, or emergent percutaneous coronary intervention (PCI).10 This tool assigns points based on patients’ age, creatinine clearance, haemoglobin, white-blood cell count, and a history prior spontaneous bleeding. The predictive performance of the PRECISE-DAPT score was tested internally and was validated in 2 external patient cohorts: the first from the PLATO trial, including 8595 patients with ACS treated with DAPT with either clopidogrel or ticagrelor, and a second cohort of 6172 patients from the BernPCI registry, a real-world all-comer patient registry.10 The PRECISE-DAPT score showed good discrimination in both validation cohorts and was ultimately evaluated among DAPT randomized patients (n = 10 081) to assess its value in discriminating net benefit for longer (12-24 months) vs shorter (3-6 months) DAPT duration.
Patients stratified based on PRECISE-DAPT and carrying a high score (score ≥ 25) had a significant increase in bleeding after a longer DAPT course, without gaining any reduction in ischemic events.10 A longer DAPT treatment in this subgroup resulted in 1 major bleeding every 38 patients treated. By contrast, in patients not carrying a high PRECISE-DAPT score (score < 25), extending DAPT duration was not associated with a significant excess of bleeding, but instead with a significant reduction in the composite ischemic endpoint of myocardial infarction (MI), definite stent thrombosis, stroke, and target vessel revascularization. A longer DAPT treatment in this subgroup prevented 1 ischemic event every 65 patients treated. Importantly, the results of this decision-making algorithm remained consistent when the analysis was restricted to patients presenting with ACS at the time of stent implantation, supporting the applicability of the score also in this higher risk population.10
The DAPT score, developed within the DAPT trial dataset (n = 11 648), includes 9 clinical and procedural variables to estimate the net benefit for ischemia and bleeding after 12 months of uneventful treatment with DAPT, and can inform decision-making on interrupting treatment at 12 months or extending it up to 30 months after PCI.11 Among the factors included in this algorithm, 8 were independent and exclusive predictors of ischemia (ie, congestive heart failure/low left ventricular ejection fraction, vein graft stenting, MI at presentation, prior MI or PCI, diabetes mellitus, stent diameter < 3mm, paclitaxel-eluting stent use, current cigarette smoking), and 1 factor was an independent and exclusive predictor of bleeding (ie, age). The DAPT score ranges from −2 to +10, with an excess of bleeding vs ischemic risk (DAPT score < 2), and higher scores identifying patients with an excess of ischemic vs bleeding risk (DAPT score ≥ 2). Importantly, among patients randomized to DAPT duration, those with a high DAPT score (ie, score ≥ 2) derived a greater benefit in terms of MI and stent thrombosis after a prolonged 30-month course of DAPT compared with the standard 12 month course (risk difference, −3.0%; 95% confidence interval [95%CI], −4.1% to −2.0%; P < .001) with only a modest increase in major bleeding (risk difference, 0.4%; 95%CI,−0.3% to 1.0%; P = .26).10 In turn, patients with a low DAPT score (ie, score < 2) did not derive any benefit in ischemic events from prolonging DAPT (risk difference −0.7%; 95%CI, −1.4% to 0.09%; P = .07), but showed a significant increase in major bleeding (risk difference, 1.5%; 95%CI, 0.8% to 2.3%; P < .001). Outcomes for the DAPT score were consistent in the subgroup of patients treated for acute MI at the time of the index intervention, which supports its use also among patients presenting with ACS.11
Taken together, dedicated risk scores to guide DAPT duration (ie, PRECISE-DAPT and DAPT score) can support clinical decision-making and are validated in the population presenting with ACS. Importantly, despite extensive validation, such tools can never replace case-by-case evaluation and clinical judgment, and none have yet been tested prospectively, which calls for further investigation.
DUAL PATHWAY THERAPY: BEYOND DUAL ANTIPLATELET THERAPYExtending the armamentarium of ACS treatment beyond the classic DAPT has recently been proposed and represents a possible advance in thrombocardiology. Despite more potent platelet inhibition, 9.9%-9.8% 1-year-rate of recurrent ischemic events were observed after ACS,3,4 meaning that despite optimal P2Y12 blockade there is still room for improvement by extending inhibition to alternative/additional pathways. Coagulation plays an important role in vascular thrombosis, and its persistent activation is associated with clinical outcomes. Inhibition of thrombin generation, blocking factor Xa activation with the use of nonvitamin-K oral anticoagulants has been recently proposed, suggesting a novel dual pathway antithrombotic strategy in patients with ACS. The addition of a lower dose of rivaroxaban to DAPT with aspirin and clopidogrel has been evaluated in the ATLAS-ACS 2 trial.12 A total of 15 526 patients with a recent ACS were randomized in this double-blind placebo-controlled trial to either placebo or 2 doses of rivaroxaban, 2.5 and 5mg twice daily, on top of the standard treatment with aspirin and clopidogrel. The mean treatment duration after inclusion was 13 months. Rivaroxaban significantly reduced the rate of major adverse cardiovascular events and cardiovascular mortality. At the prespecified evaluation of the single doses of rivaroxaban vs placebo, rivaroxaban 2.5mg showed the best net clinical benefit profile, reducing major acute cardiovascular events by 1.6% and significantly reducing both cardiovascular and all-cause death by 1.4 and 1.6%, respectively, but increasing thrombosis in myocardial infarction (TIMI) noncoronary artery bypass graft (CABG) related major bleeding by 1.2% on the absolute scale and by 3.5-fold on a relative scale, with no excess of fatal bleeding.12
The significant increase in major bleeding represented an element of concern that did not allow wide implementation of this strategy in clinical practice. To reduce the burden of bleeding, the GEMINI-ACS-1 trial evaluated a similar strategy excluding aspirin from the experimental arm, randomizing patients to rivaroxaban 2.5mg twice daily or aspirin on top of a treatment with P2Y12 inhibitor.13 A total of 3037 patients with ACS were included in the study. Randomized treatment was started at a median of 5.5 days after the index event and continued up to 291 days.13 The P2Y12 inhibitors used were clopidogrel 75mg once daily. (43.9%) or ticagrelor 90mg twice daily (56.1%) based on investigator discretion. The primary endpoint of TIMI non-CABG clinical significant bleeding occurred in 5% of patients in the rivaroxaban group and 5% in the aspirin group (hazard ratio [HR] 1.09; 95%CI, 0.80–1.50; P = .58). Although the trial was not powered to explore ischemic events, no differences were noted in the 2 study arms for the composite of cardiovascular death, MI, stroke, or definite stent thrombosis (HR, 1.06: 95%CI, 0.77–1.46; P = .73).13
Similar results were also observed in the COMPASS trial.14 In that study, patients with stable/stabilized cardiovascular disease, defined as the presence of coronary artery disease (ie, prior MI, multivessel coronary artery disease, prior multivessel PCI or CABG) or peripheral artery disease were included.14 In addition, patients with coronary artery disease should also have 2 additional risk factors to be included if their age was less than 65 years (ie, current smokers, diabetes mellitus, creatinine clearance < 60mL/min, heart failure or prior nonlacunar ischemic stroke more than 1 month previously). Individuals at high bleeding risk were excluded. Finally, 27 395 patients were randomized to rivaroxaban 2.5mg twice daily plus aspirin, rivaroxaban 5mg twice daily alone or aspirin alone for a mean follow-up of 23 months. The study was interrupted early due to the higher efficacy of the treatment with rivaroxaban 2.5mg plus aspirin compared with aspirin alone, which was associated with a 1.3% absolute reduction in the primary endpoint (ie, cardiovascular death, stroke or MI), and also to an absolute reduction of both cardiovascular and all-cause death by 0.5% and 0.7%, respectively, with a significant reduction in hospitalizations.14 By contrast, a treatment with rivaroxaban 5mg twice daily alone was not associated with a significant reduction in the primary endpoint.14 However, rivaroxaban treatment at both dosages was associated with an increase in major bleeding (mostly gastrointestinal), but not with fatal bleeding. Consequently, the net clinical benefit, taking into account both ischemia and bleeding, favored rivaroxaban 2.5mg plus aspirin but not rivaroxaban 5mg compared with aspirin alone.14 However, despite the accumulating evidence for rivaroxaban in patients with ACS, the results for the other nonvitamin K oral anticoagulants (NOACs) have so far been inconsistent. In the APPRAISE-II trial, the addition of apixaban 5mg twice daily on top of DAPT failed to show any benefit, and was terminated early due to a significant 2.5 fold increase in major bleeding, including intracranial and fatal bleeding.15 Similarly, in the smaller RE-DEEM trial, the association of dabigatran on top of DAPT was associated with a linear increase in bleeding events ranging from +77% up to +327% with an increasing dose of the drug.16 The study was, however, not designed to study the net clinical benefit for ischemia and bleeding of an ACS treatment strategy with dabigatran. In summary, dual pathway antithrombotic therapy may represent an alternative approach to the classic DAPT in patients with ACS, and the observed reduction of all-cause death in this population or in selected patients with stable cardiovascular disease is promising. However, the implementation in everyday clinical practice remains limited by concerns about the relative increase in bleeding and the cost of the drug, while currently the only factor Xa inhibitor approved in Europe for ACS secondary prevention is rivaroxaban 2.5mg.
CONCLUSIONSAntithrombotic therapy remains of paramount importance to reduce the burden of recurrent ischemic events after an ACS. Treatment individualization, which is advocated by international guidelines, allows targeting the optimal treatment to each patient, reducing the risk of an excessive bleeding hazard. Important decisions regarding the type and duration of the P2Y12 inhibitor should be taken carefully when selecting treatment after an ACS, while novel approaches in thrombocardiology, focusing on multiple antithrombotic pathways, are promising but have still to find their way into clinical practice.
CONFLICTS OF INTERESTS. Brugaletta declares a research grant to his institution from AstraZeneca and lecture fees from Abbott Vascular and Boston Scientific.
.