The impact on mortality of myocardial infarction (MI) compared with the specific degree of bleeding severity occurring after discharge in acute coronary syndrome is poorly characterized. Defining this relationship may help to achieve a favorable therapeutic risk-benefit balance.
MethodsUsing Cox-based shared frailty models, we assessed the relationship between mortality and postdischarge MI and bleeding severity—graded according to Bleeding Academic Research Consortium (BARC)—in 4229 acute coronary syndrome patients undergoing in-hospital coronary arteriography between January 2012 and December 2015.
ResultsBoth MI (HR, 5.8; 95%CI, 3.7-9.8) and bleeding (HR, 5.1; 95%CI, 3.6-7.7) were associated with mortality. Myocardial infarction had a stronger impact on mortality than BARC type 2 and 3a bleedings: (RRr, 3.8 and 1.9; P < .05), respectively, but was equivalent to BARC type 3b (RRr, 0.9; P = .88). Mortality risk after MI was significantly lower than after BARC type 3c bleeding (RRr, 0.25; P < .001). Mortality was higher after an MI in patients on dual antiplatelet therapy (DAPT) at the time of the event (HR, 2.9; 95%CI, 1.8-4.5) than in those off-DAPT (HR, 1.5; 95%CI, 0.7-3.4). In contrast, mortality was lower after a bleeding event in patients on-DAPT (HR, 1.6; 95%CI, 1.1-2.6) than in those off-DAPT (HR, 3.2; 95%CI, 1.7-5.8).
ConclusionsThe differential effect on mortality of a postdischarge MI vs bleeding largely depends on bleeding severity. The DAPT status at the time of MI or bleeding is a modifier of subsequent mortality risk.
Keywords
The threat of experiencing a recurrent ischemic event remains high after hospital discharge in acute coronary syndrome (ACS) patients and carries a markedly increased risk of subsequent death.1–4 Dual antiplatelet therapy (DAPT) represents the cornerstone for the prevention of recurrent ischemic complications in ACS patients.5–8 However, the benefit of DAPT is counterbalanced by higher bleeding risk,5–7 which in turn carries a noticeable risk of subsequent serious complications, including death.9–12
Some studies have compared the prognostic effect of postdischarge ischemic vs bleeding complications in ACS in a percutaneous coronary intervention (PCI) setting.9–11 However, there is still scarce data comparing the mortality risk associated with myocardial infarction (MI) vs the specific degree of bleeding severity.12 In addition, all of these studies were performed in selected patient recruited before 2010,9–12 when several breakthroughs in the management of ACS had not yet been widely used, which could limit the validity of their findings in more contemporary and unselected ACS patients.
Accordingly, in this study, using data from 2 contemporary cohorts of patients undergoing in-hospital coronary arteriography during the index ACS, we determined the relative impact on mortality of MI and bleeding severity experienced after hospital discharge.
METHODSPatient PopulationThe data analyzed in this study were obtained from a merged retrospective clinical registry including all patients consecutively admitted to the cardiology service of 2 tertiary hospitals with a primary and definitive diagnosis of ACS (n = 4840) from January 1, 2012 to December 31, 2015. Diagnosis of ACS was based on the presence of new-onset symptoms suggestive of myocardial ischemia and any of the following criteria: cardiac biomarkers above the higher normal laboratory limit, ST-segment deviation on electrocardiogram, in-hospital stress testing showing ischemia, or a known history of coronary vessel disease. The patients were classified as having ST-segment elevation MI or non—ST-segment elevation ACS (non—ST-segment elevation MI or unstable angina). Diagnosis of unstable angina required the presence of suggestive symptoms together with objective evidence of myocardial ischemia on stress testing or detection of a culprit lesion of ≥ 50% on coronary angiography, in addition to cardiac biomarkers below the higher normal laboratory limit.
For the purpose of the present study, patients who died in-hospital (n = 199) were excluded. Moreover, since our objective was to study the prognostic impact of MI and bleeding separately, we excluded patients who showed both complications during follow-up (n = 23). Patients without in-hospital coronary arteriography during the index admission (n = 187) and patients lost to follow-up (n = 202) were also excluded. Therefore, the final cohort of the present study consisted of 4229 patients.
Study Endpoint, Definitions, and Follow-upThe primary endpoint of interest for this analysis was all-cause death. We aimed to evaluate the association of all-cause mortality with both MI and the presence, as well as the severity, of bleeding. A secondary endpoint was description of the cardiovascular and noncardiovascular mortality rates according to MI and bleeding status during follow-up.
Our study only includes the first bleeding event and MI that occurred during follow-up. Adjudication of MI and bleeding events was performed by personnel unaware of the endpoints of interest for this analysis.
The ascertainment of MI and bleeding status during follow-up was carried out between September and October of 2016. We reviewed the electronic medical record and all the medical attendances and hospital records. For each patient in the study, we had information on the characteristics of bleeding events, location, clinical severity, imaging tests, hemoglobin levels, and blood transfusion.
Myocardial infarction during follow-up was defined as type 1 MI (spontaneous MI), type 3 MI (fatal MI in the absence of biomarker values), or type 4b MI (MI related to stent thrombosis), in accordance with the Third Universal Definition.13
Bleeding events were classified according to the Bleeding Academic Research Consortium (BARC) criteria.14 Due to the retrospective nature of the study and the difficulty involved in the adjudication of BARC bleeding type 1, this type of bleeding was not included in the present study. Because the primary outcome of this analysis was mortality, bleeding events belonging to class 5 of BARC-defined bleeding (fatal bleeding) were not analyzed as a separate class but were distributed to other classes according to initial assessment, as previously described.15,16 Therefore, the BARC hemorrhages analyzed in this study were type 2, 3a, 3b, and 3c.
The cause of death (cardiovascular vs noncardiovascular) was classified according to the definitions of Hicks et al.17 When no information was available or there was no consensus on the cause of death, it was included in the “unknown or unclassifiable cause of death” group.
Statistical AnalysisBaseline and clinical characteristics were summarized by MI and bleeding status experienced after hospital discharge. Continuous variables are presented as medians [interquartile ranges], and categorical variables as counts and proportions. Differences between continuous variables in patients who did and did not experience bleeding events or MIs were compared using the Wilcoxon rank-sum test. The chi-square test was used to compare categorical variables.
Since MI and bleeding status change over time, the adjusted effect of postdischarge MI and bleeding on subsequent all-cause mortality was investigated using separate Cox-type shared frailty models with MI and bleeding as time-varying covariates. For the prior purpose, we used the user-written command “strmcure” (without cured fraction and with nonstratification), which allows testing a direct and indirect effect of a baseline exposure on the terminal endpoint.18 In the first “strmcure” model, we investigated the effect of postdischarge MI and bleeding as a whole. In another model, on top of postdischarge MI, the severity of BARC type bleeding events was entered as a hierarchical time-varying covariate in which the highest risk state was BARC type 3c followed by BARC type 3b, BARC type 3a, and then BARC type 2 bleeding.
To obtain evidence on the role of DAPT status at the time of MI or bleeding on mortality risk, an additional analysis was performed by generating categorical time-dependent covariates for both MI and bleeding with different levels corresponding to whether a patient was on- or off-DAPT at the time of each respective event.
All regression models were adjusted using covariates that were either significantly different between groups according to MI and bleeding status, or plausibly related to mortality. Continuous variables that did not meet linearity assumptions were transformed. The following covariates were included in the regression models: age ≥ 65 years, female sex, body mass index (kg/m2), current smoking, hypertension, dyslipidemia, diabetes mellitus, prior coronary artery disease, history of congestive heart failure or left ventricular ejection fraction < 40%, prior vascular disease, history of bleeding, malignant disease, chronic obstructive pulmonary disease, ACS type, baseline serum creatinine (mg/dL) and hemoglobin values (g/dL), multivessel coronary disease, in-hospital PCI, in-hospital coronary artery bypass graft, treatment at discharge, and study center.
The bootstrap method (500 iterations) was used to calculate and estimate statistical significance and hazard ratios (HRs) and their 95% confidence intervals (95%CIs).
To estimate the number of deaths attributable to MI or bleeding, the adjusted HR for each event was applied to the actual number of deaths according to the following formula: number of deaths among patients with MI or bleeding × [adjusted HR-1]/ adjusted HR.
The differential impact on mortality of MI vs each BARC bleeding type being studied was generated from the corresponding adjusted Cox-based models using the lincom command and is expressed as relative risk ratio (RRr).
Finally, we investigated the time-pattern of the risk of death associated with postdischarge MI and bleeding as a function of the time elapsed since the event. For this purpose, the functional form of the relationship between the adjusted hazard of death, and the time elapsed since bleeding or MI was fitted using fractional polynomial, and the resulting curve along with the 95%CI of the mean was plotted.
Values were considered statistically significant when the P value was < .05. All statistical analyses were performed using Stata/MP 13.1.
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local ethics committee.
RESULTSEvents During Follow-upDuring a median follow-up of 455 days [377-783] days, 204 patients experienced a MI (crude rate: 3.1 per 100 person-years; 95%CI, 2.65-3.49). The median time from discharge to the MI event was 192 [56-340] days. Most postdischarge MIs were non—ST-segment elevation MIs (83.8%). Twelve MIs were fatal, with ST-segment elevation MI accounting for 62.8% of the case-fatality.
A total of 500 patients had a bleeding complication (crude rate: 7.7 per 100 person-years; 95%CI, 7.01-8.36), with a distribution according to BARC type 2, 3a, 3b and 3c bleeding event of 359 (8.5%), 61 (1.4%), 49 (1.2%), and 31 (0.7%) patients, respectively.
The median time from discharge to BARC type 2, 3a, 3b, and 3c bleeding events was 300 [117-405] days, 149 [41-410] days, 167 [82-321] days, and 314 days [177-622] days, respectively.
Overall, 91.2% of the bleedings were spontaneous (ie, nonprocedural-related). Around half (53%) of bleeding episodes were gastrointestinal and 9 bleeding events were fatal with intracranial bleeding accounting for 55.6% of the case-fatality.
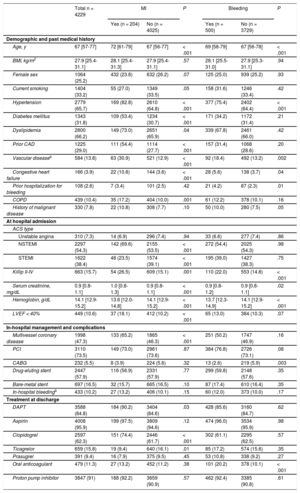
Baseline and clinical characteristics by postdischarge MI and bleeding status are presented in Table 1. In summary, patients who had a postdischarge MI were older and in general had a greater comorbidity burden (hypertension, diabetes, dyslipidemia, prior vascular disease, heart failure, and chronic obstructive pulmonary disease) compared with patients who did not have a MI. They were also more likely to have multivessel coronary artery disease, but had similar a rate of in-hospital PCI compared with patients without postdischarge MI, and they were more likely to receive clopidogrel at hospital discharge.
Baseline and Clinical Characteristics by Postdischarge MI and Bleeding Status
| Total n = 4229 | MI | P | Bleeding | P | |||
|---|---|---|---|---|---|---|---|
| Yes (n = 204) | No (n = 4025) | Yes (n = 500) | No (n = 3729) | ||||
| Demographic and past medical history | |||||||
| Age, y | 67 [57-77] | 72 [61-79] | 67 [56-77] | < .001 | 69 [58-79] | 67 [56-78] | < .001 |
| BMI, kg/m2 | 27.9 [25.4-31.1] | 28.1 [25.4-31.3] | 27.9 [25.4-31.1] | .57 | 28.1 [25.5-31.0] | 27.9 [25.3-31.1] | .94 |
| Female sex | 1064 (25.2) | 432 (23.8) | 632 (26.2) | .07 | 125 (25.0) | 939 (25.2) | .93 |
| Current smoking | 1404 (33.2) | 55 (27.0) | 1349 (33.5) | .05 | 158 (31.6) | 1246 (33.4) | .42 |
| Hypertension | 2779 (65.7) | 169 (82.8) | 2610 (64.8) | < .001 | 377 (75.4) | 2402 (64.4) | < .001 |
| Diabetes mellitus | 1343 (31.8) | 109 (53.4) | 1234 (30.7) | < .001 | 171 (34.2) | 1172 (31.4) | .21 |
| Dyslipidemia | 2800 (66.2) | 149 (73.0) | 2651 (65.9) | .04 | 339 (67.8) | 2461 (66.0) | .42 |
| Prior CAD | 1225 (29.0) | 111 (54.4) | 1114 (27.7) | < .001 | 157 (31.4) | 1068 (28.6) | .20 |
| Vascular diseasea | 584 (13.8) | 63 (30.9) | 521 (12.9) | < .001 | 92 (18.4) | 492 (13.2) | .002 |
| Congestive heart failure | 166 (3.9) | 22 (10.8) | 144 (3.6) | < .001 | 28 (5.6) | 138 (3.7) | .04 |
| Prior hospitalization for bleeding | 108 (2.6) | 7 (3.4) | 101 (2.5) | .42 | 21 (4.2) | 87 (2.3) | .01 |
| COPD | 439 (10.4) | 35 (17.2) | 404 (10.0) | .001 | 61 (12.2) | 378 (10.1) | .16 |
| History of malignant disease | 330 (7.8) | 22 (10.8) | 308 (7.7) | .10 | 50 (10.0) | 280 (7.5) | .05 |
| At hospital admission | |||||||
| ACS type | |||||||
| Unstable angina | 310 (7.3) | 14 (6.9) | 296 (7.4) | .94 | 33 (6.6) | 277 (7.4) | .86 |
| NSTEMI | 2297 (54.3) | 142 (69.6) | 2155 (53.5) | < .001 | 272 (54.4) | 2025 (54.3) | .98 |
| STEMI | 1622 (38.4) | 48 (23.5) | 1574 (39.1) | < .001 | 195 (39.0) | 1427 (38.3) | .75 |
| Killip II-IV | 663 (15.7) | 54 (26.5) | 609 (15.1) | .001 | 110 (22.0) | 553 (14.8) | < .001 |
| Serum creatinine, mg/dL | 0.9 [0.8-1.1] | 1.0 [0.8-1.3] | 0.9 [0.8-1.1] | < .001 | 0.9 [0.8-1.2] | 0.9 [0.8-1.1] | .02 |
| Hemoglobin, g/dL | 14.1 [12.9-15.2] | 13.6 [12.0-14.8] | 14.1 [12.9-15.2] | < .001 | 13.7 [12.3-14.9] | 14.1 [12.9-15.2] | < .001 |
| LVEF < 40% | 449 (10.6) | 37 (18.1) | 412 (10.2) | < .001 | 65 (13.0) | 384 (10.3) | .07 |
| In-hospital management and complications | |||||||
| Multivessel coronary disease | 1998 (47.3) | 133 (65.2) | 1865 (46.3) | < .001 | 251 (50.2) | 1747 (46.9) | .16 |
| PCI | 3110 (73.5) | 149 (73.0) | 2961 (73.6) | .87 | 384 (76.8) | 2726 (73.1) | .08 |
| CABG | 232 (5.5) | 8 (3.9) | 224 (5.6) | .32 | 13 (2.6) | 219 (5.9) | .003 |
| Drug-eluting stent | 2447 (57.9) | 116 (56.9) | 2331 (57.9) | .77 | 299 (59.8) | 2148 (57.6) | .35 |
| Bare-metal stent | 697 (16.5) | 32 (15.7) | 665 (16.5) | .10 | 87 (17.4) | 610 (16.4) | .35 |
| In-hospital bleedingb | 433 (10.2) | 27 (13.2) | 406 (10.1) | .15 | 60 (12.0) | 373 (10.0) | .17 |
| Treatment at discharge | |||||||
| DAPT | 3588 (84.8) | 184 (90.2) | 3404 (84.6) | .03 | 428 (85.6) | 3160 (84.7) | .62 |
| Aspirin | 4008 (95.9) | 199 (97.5) | 3809 (94.6) | .12 | 474 (96.0) | 3534 (95.9) | .98 |
| Clopidogrel | 2597 (62.3) | 151 (74.4) | 2446 (61.7) | < .001 | 302 (61.1) | 2295 (62.5) | .57 |
| Ticagrelor | 659 (15.8) | 19 (9.4) | 640 (16.1) | .01 | 85 (17.2) | 574 (15.6) | .35 |
| Prasugrel | 391 (9.4) | 16 (7.9) | 375 (9.5) | .45 | 53 (10.8) | 338 (9.2) | .27 |
| Oral anticoagulant | 479 (11.3) | 27 (13.2) | 452 (11.2) | .38 | 101 (20.2) | 378 (10.1) | < .001 |
| Proton pump inhibitor | 3847 (91) | 188 (92.2) | 3659 (90.9) | .57 | 462 (92.4) | 3385 (90.8) | .61 |
ACS, acute coronary syndrome; BARC, Bleeding Academic Research Consortium; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non—ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as No. (%) or median [interquartile range].
Patients who had a bleeding event after discharge were older, had a greater comorbidity burden (hypertension, prior vascular disease, and heart failure), and more frequently had prior bleeding than patients who did not have a bleed. They were also less likely to undergo in-hospital coronary artery bypass graft, and to more frequently receive oral anticoagulant at hospital discharge.
During follow-up, there were 335 deaths; the number of patients who died following MI or bleeding is summarized in Table 2. When considered as time-varying covariates in the multivariable-adjusted model, both MI (HR, 5.8; 95%CI, 3.7-9.8) and bleeding (as a whole) (HR, 5.1; 95%CI, 3.6-7.7) were significantly associated with mortality. The mortality attributable to MI was 10.6%, while bleeding-attributable mortality was 16.8%.
Crude Mortality Rate by Postdischarge Myocardial Infarction and Bleeding Status and Their Effect (Hazard Ratio) on Subsequent All-cause Mortality
| Event | No. of patients | No. of deaths | Crude mortality rate (per 100 person-years) (95%CI) | Adjusted HRa (95%CI) | P | Attributable mortalityb No. (%) |
|---|---|---|---|---|---|---|
| Neither event | 3525 | 222 | 3.5 (3.1-4.0) | — | — | — |
| MI | 204 | 43 | 20.7 (15.4-27.9) | 5.8 (3.7-9.8) | < .001 | 36 (10.6) |
| Bleeding | 500 | 70 | 18.4 (14.5-23.2) | 5.1 (3.6-7.7) | < .001 | 56 (16.8) |
95%CI, 95%confidence interval; ACS, acute coronary syndrome; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Adjusted for: age ≥ 65 years, female sex, body mass index (kg/m2), current smoking, hypertension, dyslipidemia, diabetes mellitus, prior coronary artery disease, history of congestive heart failure or LVEF < 40%, prior vascular disease, history of bleeding, malignant disease, COPD, ACS type, baseline serum creatinine (mg/dL) and hemoglobin (g/dL) values, multivessel coronary disease, in-hospital PCI, in-hospital coronary artery bypass graft, treatment at discharge, and study center.
Detailed univariable and multivariable Cox analyses are shown in and .
When we repeated this analysis and included only those patients who received DAPT at discharge (n = 3588 with: 189 MIs, 428 bleeding events, and 270 deaths), the results did not substantially change: HR, 5.7; 95%CI, 3.6-9.3 for MI, and HR, 5.4; 95%CI, 3.6-8.1) for bleeding ().
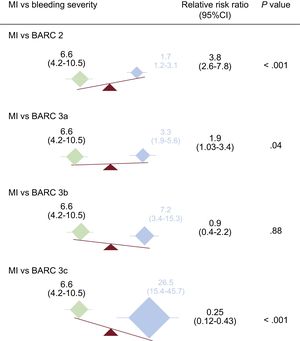
The adjusted differential effect on mortality of MI and each of the 4 BARC severity categories is shown in Figure 1. There was a stepwise increase in adjusted mortality risk from the least to the most severe BARC bleeding category. The relative hazard of death was 4-fold higher in patients experiencing MI vs BARC type 2 bleeding [6.6 vs 1.7; RRr, 3.8; 95%CI, 2.6–7.8; P < .001], and almost 2-fold higher in patients experiencing MI vs BARC type 3a bleeding (6.6 vs 3.3; RRr, 1.9; 95%CI, 1.03-3.4; P = .04). In contrast, no significant difference was found comparing MI and BARC type 3b bleeding (6.6 vs 7.2; RRr, 0.9; 95%CI, 0.4–2.2; P = .88). However, the risk of mortality associated with MI was significantly lower (4-fold) than for BARC type 3c bleeding (HR, 26.5 vs 6.6; RRr, 0.25; 95%CI, 0.12–0.43; P < .001).
When we rerun the prior analysis and included only the 3588 patients who received DAPT at discharge, we found similar results to those observed in the entire cohort ().
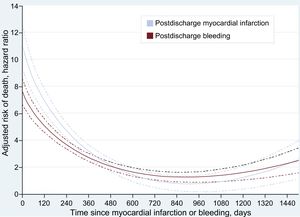
The adjusted mortality risk following both MI and bleeding peaked early after the occurrence of these events, and despite showing a substantial deceleration thereafter, remained significant for several months (Figure 2). A similar pattern was observed in patients discharged with DAPT ().
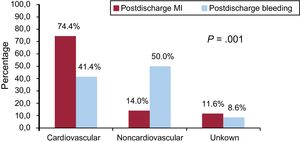
The overall rate of cardiovascular death was 45.4% (n = 152/335), and the cause of death was unknown or unclassifiable in 47 (14%) patients. The rate of cardiovascular death in patients with MI was 74.4% (n = 32/43) vs 41.4% (n = 29/70) in patients who had postdischarge bleeding (P < .001) (Figure 3). Similar rates of unknown or unclassifiable causes of death were observed after MI (11.6%) and bleeding (8.6%) during follow-up (P = .6). The specific cause of death by MI and bleeding events is shown in .
The influence of DAPT status at the time of postdischarge MI and bleeding on mortality is shown in . Among the 3588 patients discharged with DAPT, there were 189 MIs, 428 bleeding events, and 270 deaths (crude mortality rate = 3.2 per 100 person-years; 95%CI, 2.8-3.7).
Data on DAPT status at the time of MI or bleeding were available in all except 12 patients (2 MIs and 10 bleeding events). Among patients with an MI event, 73% (n = 138/189) were on-DAPT. Of those patients who had a bleeding event, 74.8% (n = 320/428) were on-DAPT.
The mortality risk after MI was almost 3-fold higher for patients on-DAPT (HR, 2.9; 95%CI, 1.8-4.5) than for those off-DAPT (HR, 1.5; 95%CI, 0.7-3.4). In contrast, mortality risk associated with bleeding was ∼2-fold lower for patients on-DAPT (HR, 1.6; 95%CI, 1.1-2.6) than for off-DAPT patients (HR, 3.2; 95%CI, 1.7-5.8) (Table 3).
Effect of Postdischarge MI and Bleeding on Subsequent Mortality Stratified by Dual Antiplatelet Therapy Status at the Time of Each Respective Eventa
| Death, No. | Crude mortality rate (per 100 person-years) (95%CI) | Adjusted HR (95%CI)b | P | |
|---|---|---|---|---|
| MI (n = 189) | ||||
| On-DAPT (n = 138) | 31 | 19.7 (14.0-27.7) | 2.9 (1.8-4.5) | < .001 |
| Off-DAPT (n = 49) | 7 | 10.7 (5.1-22.4) | 1.5 (0.7-3.4) | .30 |
| Missing data on DAPT status (n = 2) | 1 | --- | --- | --- |
| Bleeding (n = 428) | ||||
| On-DAPT (n = 320) | 36 | 9.0 (6.4-12.7) | 1.6 (1.1-2.6) | .03 |
| Off-DAPT (n = 98) | 20 | 13.4 (8.5-21.0) | 3.2 (1.7-5.8) | < .001 |
| Missing data on DAPT status (n = 10) | 2 | --- | --- | --- |
95%CI, 95% confidence interval; ACS, acute coronary syndrome; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Age ≥ 65 years, female sex, body mass index (kg/m2), current smoking, hypertension, dyslipidemia, diabetes mellitus, prior coronary artery disease, history of congestive heart failure or LVEF < 40%, prior vascular disease, history of bleeding, malignant disease, COPD, ACS type, baseline serum creatinine (mg/dL) and hemoglobin (g/dL) values, multivessel coronary disease, in-hospital PCI, in-hospital coronary artery bypass graft, treatment at discharge, and study center.
In this contemporary cohort, we observed several relevant findings. First, we found that the risk of mortality following an MI was significantly higher than after a BARC type 2 and 3a bleeding, being similar after a BARC type 3b bleeding. In contrast, mortality risk was significantly higher after a BARC type 3c bleeding than after an MI.
Second, we confirm that the temporal relationship between the risk of death after an MI or bleeding is similar, characterized by reaching an early peak after the event followed by a substantial deceleration thereafter, remaining significantly high for several months. Third, we observed that patients experiencing an MI after discharge died more frequently from cardiovascular causes, in contrast to patients experiencing a postdischarge bleeding event, who died predominantly from noncardiovascular causes. Fourth, we found that DAPT status at the time of MI or bleeding was a modifier of mortality risk. While the mortality hazard associated with postdischarge MI was higher among patients on-DAPT compared with those off-DAPT, there was an opposite result with postdischarge bleeding, with a higher mortality risk among those off-DAPT than those on-DAPT.
This is the first study in a contemporary and nonselected cohort of patients with ACS, comparing the effect on mortality of postdischarge MI and bleeding severity, and assessing the influence of DAPT status on mortality at the time of these events. Several studies have examined the associations of mortality between thrombotic or hemorrhagic complications after discharge for an ACS; however, very few reports have compared the prognostic impact of these complications in a vis-á-vis fashion.
A retrospective analysis performed by Kazi et al.,10 based on an administrative claims database, showed comparable mortality risks associated with postdischarge MI (HR, 1.9) and bleeding (HR, 1.6) in patients undergoing percutaneous coronary intervention between 1996 and 2008. However, in that study, the definition of bleeding was bleeding requiring hospitalization, events were not graded according to the severity of bleeding, and data on important mortality predictors such as coronary anatomy and left ventricular ejection fraction were not available.
In a post hoc analysis of the PARIS registry, Baber et al.,11 reported a similar mortality risk associated with MI or stent thrombosis (HR, 3.3) and bleeding (BARC type 2 and 3) (HR, 3.5) over a 2-year period in 5018 patients undergoing PCI. However, the bleeding events considered were only BARC type 2 and 3, avoiding the severity of BARC type 3 bleeding (BARC 3a, 3b, and 3c). Additionally, this study included only nonfatal events, which might have influenced the effect of ischemic or hemorrhagic events on mortality. Moreover, the PARIS study was performed before 2011, less than 45% of the patients had an ACS, and almost all participants were treated with clopidogrel (∼6% with prasugrel).
A third study was based on the TRACER clinical trial population. In this interesting study, Valgimigli et al.,12 found that the risk of death after an MI was higher than the risk of death associated with BARC type 2 and BARC type 3a, being similar to BARC type 3b, and was only exceeded by BARC 3c bleeding. However, the previous study included patients with non—ST-segment elevation ACS exclusively, all participants were recruited before 2011, no patients were treated with prasugrel or ticagrelor, and the influence on mortality of DAPT at the time of MI and bleeding was not addressed.
Therefore, the findings of these 3 studies may not be generalizable to all-ACS patients or to contemporary practice.
With our cohort, we confirm the findings of Valgimigli et al.12 and expand the knowledge to all-ACS patients. The influence on mortality of DAPT at the time of MI and bleeding occurrence was also investigated in the present cohort. We observed a higher mortality risk after an MI in patients who were on- vs off-DAPT. Conversely, we observed a lower mortality risk after a bleeding event in patients who were on- vs off-DAPT. These results matched those reported in the PARIS population with PCI patients.11 The excess risk of death in patients who had an MI despite being on-DAPT may be explained by the presence of a strong prothrombotic milieu or therapeutic nonadherence, or might be a marker of a more severely ill population. Irrespective of these possibilities, the association between ongoing DAPT use and increased risk of death after an MI suggests that there may still be a role for more intensive antithrombotic therapy at least in some of these patients.
Of note, the reduced number of deaths occurring after MI among off-DAPT patients in our study makes the results speculative and requires confirmation in larger cohorts.
Appreciably, the higher mortality associated with an episode of bleeding while off-DAPT may be related not only to the interruption of DAPT but also to discontinuation of other medications known to improve the prognosis of ACS patients, and/or could be a marker of higher risk patients (ie, confounding by indication) not fully captured in the multivariable analyses. The lower mortality risk among on-DAPT bleeders vs off-DAPT bleeders might suggest that there may still be a role for DAPT after bleeding events, and may represent a need for more precise tailoring of clinical decisions in relation to the discontinuation of DAPT after a bleed.
Interestingly, in our study, mortality risk following MI and bleeding peaked early, and substantially declined thereafter. This finding is of clinical interest since it could help to identify critical periods after these events. In addition, the sustained effect on mortality for several months after an MI or bleeding could help to clarify contrasting results published by others.9,11,19 In one study based on all-ACS patients, the impact of bleeding was only confined to the first 40 days after the event.9 In a second report including ST-segment elevation MI patients, recurrent MI impacted mortality for > 1 year, whereas the effect of severe bleeding disappeared 30 days after the event.19 Recently, Baber et al.,11 found that the effect of bleeding on mortality was sustained over 2 years after the event, in contrast to the effect of an MI that was no longer significant after the first year. Last, in a study by Valle et al.,20 postdischarge bleeding remained associated with death after a median follow-up of 665 [375-730] days. However, it is important to be aware that reconciling these inconsistent results is challenging as they concern different populations, different types and definitions of events, and different statistical methods.
Importantly, in this study we also report the timing of postdischarge MI and bleeding. Defining the periods of higher vulnerability for MI and bleeding after discharge for an ACS could help to focus physicians’ attention on these periods, allowing optimization of preventive efforts and resources.
Taken all together, our results provide insights to better understand the risk of MI and bleeding after discharge for an ACS, and their differential prognostic impact on mortality. Our findings demonstrate the need for caution with patients at risk for postdischarge MI and bleeding. They deserve close follow-up and evaluation since MI and bleeding occur early on and carry a substantial risk of death. DAPT reduces postdischarge MI risk in ACS while its benefit is counterbalanced by higher bleeding risk. Our study shows important aspects that could help in the design of the optimal DAPT strategy in order to achieve a favorable risk-benefit balance.
LimitationsThis was a retrospective study with the limitations inherent to this type of design. One important limitation was the lack of some data on follow-up in 202 patients who were excluded from the main analysis. Although this could theoretically result in selection bias, a comparison of patients with and without missing data on follow-up indicated no significant differences between the 2 groups (). Additionally, although every effort was made to collect MI and bleeding events during follow-up, we cannot completely rule out the possibility that the rate of MI was underestimated. Furthermore, we did not collect data on BARC type 1 and type 4 bleeding. However, this could represent a mild limitation, since in several studies, BARC type 1 bleeding was not associated with a significant increase in the risk of death, and postdischarge BARC type 4 is a rarity as very few patients during follow-up underwent coronary artery bypass graft.
In this study, data on MI type 2 (MI secondary to an ischemic imbalance), MI type 4a (MI related to PCI), and MI type 5 (MI related to coronary artery bypass graft) were not collected; hence our findings cannot be generalized to all types of MI. In addition, we did not perform subgroup analysis according to the P2Y12 inhibitor due to the relatively small sample size; therefore, the results cannot be generalized to the different P2Y12 inhibitor types.
Finally, we were not able to characterize the prognosis of patients with repeated MIs or bleeding events since we only had data of the first MI and bleeding complication during follow-up. Therefore, our results cannot be generalized to this particular group of patients, which, based on previous studies, could represent around 1% of the ACS population.12
CONCLUSIONSThe differential effect of a postdischarge MI compared with a postdischarge bleeding on mortality largely depended on the severity of bleeding. MI impacted mortality to a greater extent than BARC type 2 and BARC type 3a bleeding, the mortality risk being similar for MI and BARC type 3b bleeding. In contrast, BARC type 3c bleedings carried higher mortality risks than MIs. The time-pattern of the associated hazard of mortality was similar between MI and bleeding events. DAPT status at the time of MI or bleeding was a modifier of subsequent mortality risk. These findings may help to depict the risk and benefit profile of antithrombotic medications.
CONFLICTS OF INTERESTE. Abu-Assi is Associate Editor of Revista Española de Cardiología.
- –
Postdischarge MI and bleeding increase the risk of subsequent mortality.
- –
Little is known about the magnitude of mortality risk after an MI vs the specific degree of bleeding severity in contemporary unselected ACS patients.
- –
The influence of DAPT on mortality status at the time of MI and bleeding occurring after discharge for an ACS is currently poorly characterized.
- –
The risk of mortality following an MI was higher than after a BARC type 2 and 3a bleeding event, being equivalent to BARC type 3b.
- –
The risk of death after a BARC type 3c bleeding event is significantly higher than after an MI.
- –
An MI occurring while on- vs off-DAPT conferred a higher mortality risk. Conversely, a bleeding event while on- vs off-DAPT was related to a lower mortality risk.
- –
The cause of death after an MI was mainly cardiovascular in contrast to the cause of death after a bleeding event, which was mainly noncardiovascular.