Contemporary data on the incidence and prognosis of heart failure (HF) and the influence of left ventricular ejection fraction (LVEF) in the setting of acute coronary syndrome (ACS) are scant. The aim of this study was to examine the relationship between LVEF and HF with long-term prognosis in a cohort of patients with ACS.
MethodsThis is a retrospective observational study of 6208 patients consecutively admitted for ACS to 2 different Spanish hospitals. Baseline characteristics were examined and a follow-up period was established for registration of death and HF rehospitalization as the primary endpoint.
ResultsAmong the study participants, 5064 had ACS without HF during hospitalization: 290 (5.8%) had LVEF<40%, 540 (10.6%) LVEF 40% to 49%, and 4234 (83.6%) LVEF ≥ 50%. The remaining 1144 patients developed HF in the acute phase: 395 (34.6%) had LVEF<40%, 251 (21.9%) LVEF 40% to 49%, and 498 (43.5%) LVEF ≥ 50%. Patients with LVEF 40% to 49% had a demographic and clinical profile with intermediate features between the LVEF <40% and LVEF ≥ 50% groups. Kaplan-Meier curves showed that mortality and HF readmissions were statistically different depending on LVEF in the non-HF group but not in the HF group. Left ventricular ejection fraction ≥ 50% was an independent prognostic factor in the non-HF group only.
ConclusionsIn ACS, long-term prognosis is considerably worse in patients who develop HF during hospitalization than in patients without HF, irrespective of LVEF. This parameter is a strong prognostic predictor only in patients without HF.
Keywords
Heart failure (HF) and acute coronary syndromes (ACS) are the main causes of death and hospitalization in industrialized countries.1 It is well known that the presence of HF during ACS is one of the most important clinical manifestations leading to adverse outcomes.2 Although improvements in the treatment of ACS over the past decade have reduced short-term mortality, HF complicating ACS is still challenging because it is associated with a high 1-year mortality risk.3,4
Current practice guidelines recommend the measurement of left ventricular ejection fraction (LVEF) after an ACS5 because between one-third and a half of patients who present with an ACS are discharged with left ventricular systolic dysfunction.6 Reduced LVEF is a strong predictor of mortality and rehospitalization7–11 and recent studies suggest that patients with HF with preserved LVEF complicating ACS have only a slightly better prognosis than patients with reduced LVEF.12
The new 2016 European Society of Cardiology (ESC) HF Guidelines propose a new classification according to the level of LVEF, as follows: reduced LEVF is <40%, midrange LVEF ranges from 40% to 49%, and preserved LVEF is ≥ 50%.13 Currently, there is limited information on the clinical, prognostic, and therapeutic implications of this classification.14
The aim of our study was to analyze the relationship between the LVEF and HF with long-term prognosis in a cohort of patients with ACS. We also aimed to determine the prognostic implications of the new LVEF classification proposed by the ESC-HF guidelines.
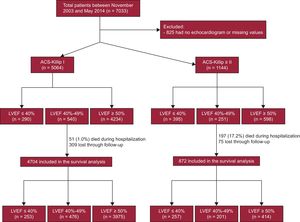
METHODSStudy DesignWe designed a retrospective study of consecutive patients admitted to the coronary care unit and the hospitalization ward of 2 Spanish hospitals due to ACS from November 2003 through May 2014 (n=7033). The contemporary cohort was comprehensive and the only exclusion criterion was the absence of available LVEF data or missing values during the index hospitalization (825 patients); the final cohort was based on 6208 patients. The study protocol and the review of the clinical history was approved by the ethics committee of the coordinating hospital.
Variables DefinitionPatients were classified as having ST-segment elevation myocardial infarction (STEMI) or non—ST-segment elevation ACS that included unstable angina and non—STEMI. The diagnosis of myocardial infarction was made according to the 2012 ESC definition.15 Diagnosis of unstable angina required the presence of suggestive symptoms together with objective evidence of myocardial ischemia on stress testing or detection of a culprit lesion of 50% on coronary angiography, in addition to cardiac biomarkers below the upper normal laboratory limit.15 Both centers are involved in regional STEMI systems of care and primary angioplasty, the strategy of choice within the inclusion period, was performed in> 90% of STEMI patients in both centers.
Risk factors, clinical antecedents, treatments, complementary tests, and main diagnosis at discharge were collected from all patients by trained medical staff. The diagnostic and therapeutic ACS protocols at both centers included blood sample determinations in the emergency room and in fasting state after hospital admission. We identified patients with prior coronary artery disease by searching for those who already had a clinical diagnosis of myocardial infarction, and/or a history of angina or angina-driven coronary revascularization. Prior HF was identified if patients had at least 1 hospitalization in which HF was the main diagnosis or if they had typical signs and symptoms consistent with HF syndrome along with compatible imaging studies (X-ray or echocardiogram).
We defined index HF as the presence of pulmonary rales or the use of intravenous diuretics or intravenous inotropic drugs during ACS admission and described the level of HF severity by Killip class.16 The highest class observed during the first 7 days of hospital stay was used in the present analysis. Two-dimensional transthoracic echocardiography was performed in all participants by a level III-certified echocardiographer as part of routine clinical practice during hospitalization and LVEF was assessed according to the international Simpson method17 at discharge.
Patient ClassificationThe final population included 6208 patients. They were divided into 2 groups depending on the development of HF (Killip=1 or Killip ≥ 2). In each group, the patients were classified according to LVEF following the current HF-ESC guidelines cutoff13 (Figure 1).
Follow-up and Outcome MeasuresWe used a well-established protocol for postdischarge follow-up: after discharge, patients were followed up in a monographic consultation of ischemic heart disease and primary care. The structured follow-up was carried out through the electronic history by reviewing all medical assistance and hospital records and resorting in certain cases to telephone contact.
Primary endpoints were all-cause mortality and HF after hospital discharge. The development of HF on follow-up was identified if the patient had at least 1 hospitalization with HF as the main diagnosis.
Statistical AnalysisQuantitative variables are presented as mean±standard deviation and differences were assessed by the Student t test or ANOVA test. Qualitative variables are presented as percentages and differences were analyzed by the chi-square test. Multicollinearity between LVEF and HF during hospitalization, revascularization and age was rejected since variance inflation factors were low.
All-cause mortality after hospital discharge was assessed by survival analyses. The observed event risk was calculated as a Kaplan-Meier estimate using the log rank test.
All-cause mortality predictors were assessed by Cox regression models, after verification of the proportional risk assumption by the Schoenfeld residuals test, using all variables that obtained P values <.1 in the univariate analysis or could have plausible clinical implication; the results are presented as hazard ratios (HR) and 95% confidence intervals (95%CI). The first model included all the variables and, after identification of a positive interaction between HF and LVEF categories, a second model was carried out stratified by HF during the index admission. The discriminative and calibration ability of survival models were assessed by means of Harrell's C-statistic and the Gronnesby and Borgan test, respectively. The incidence of HF could be affected by patient death and, therefore, the usual techniques for time-to-event analysis would provide biased or uninterpretable results due to the presence of competing risks. To avoid such effects, we applied the model introduced by Fine and Gray18 to test the competing events. The incidence of HF is presented in cumulative incidence function graphs and results of the multivariate analysis as a subhazard ratio (sHR). Harrell's C-statistic test was used to assess the model's discrimination while calibration was tested by the Gronnesby and Borgan test. Patients lost during follow-up were categorized as missing, as well as those who lacked any of the main variables for the analyses, although these were very few.
Statistical difference was accepted at P <.05. All analyses were performed using STATA 14.2 (StataCorp, 2009, Stata Statistical Software, Release 14, College Station, TX, StataCorp LP).
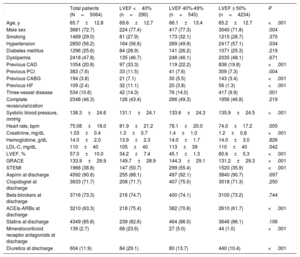
RESULTSCharacteristics of Patients Without Heart Failure According to Left Ventricular Ejection FractionA total of 5064 patients had an ACS without HF and were assigned to 1 of 3 groups according to LVEF: 290 (5.7%) with LVEF <40%, 540 (10.7%) with LVEF 40% to 49%, and 4234 (83.6%) with LVEF ≥ 50%. The characteristics of the patients are shown in Table 1. Age, history of hypertension, coronary artery disease, previous revascularization (surgical or percutaneous), previous HF, renal function, blood pressure, heart rate, STEMI, treatment with angiotensin-converting enzyme inhibitors (ACEIs)-angiotensin receptor blockers, mineralocorticoid receptor antagonists, and diuretics were statistically significantly different between groups.
Characteristics of Patients With Acute Coronary Syndrome and Without Heart Failure as a Function of Left Ventricular Ejection Fraction
| Total patients (N=5064) | LVEF <40% (n=290) | LVEF 40%-49% (n=540) | LVEF ≥ 50% (n=4234) | P | |
|---|---|---|---|---|---|
| Age, y | 65.7±12.8 | 69.6±12.7 | 66.1±13.4 | 65.2±12.7 | <.001 |
| Male sex | 3681 (72.7) | 224 (77.4) | 417 (77.3) | 3040 (71.8) | .004 |
| Smoking | 1469 (29.0) | 81 (27.9) | 173 (32.1) | 1215 (28.7) | .370 |
| Hypertension | 2850 (56.2) | 164 (56.6) | 269 (49.9) | 2417 (57.1) | .034 |
| Diabetes mellitus | 1296 (25.6) | 84 (28.9) | 141 (26.2) | 1071 (25.3) | .219 |
| Dyslipemia | 2418 (47.8) | 135 (46.7) | 248 (46.1) | 2035 (48.1) | .671 |
| Previous CAD | 1054 (20.8) | 97 (33.3) | 119 (22.2) | 838 (19.8) | <.001 |
| Previous PCI | 383 (7.6) | 33 (11.5) | 41 (7.6) | 309 (7.3) | .004 |
| Previous CABG | 194 (3.8) | 21 (7.1) | 30 (5.5) | 143 (3.4) | <.001 |
| Previous HF | 109 (2.4) | 32 (11.1) | 20 (3.8) | 56 (1.3) | <.001 |
| Three-vessel disease | 534 (10.6) | 42 (14.3) | 76 (14.0) | 417 (9.9) | .001 |
| Complete revascularization | 2348 (46.3) | 126 (43.4) | 266 (49.3) | 1956 (46.8) | .219 |
| Systolic blood pressure, mmHg | 138.3±24.6 | 131.1±24.1 | 133.6±24.3 | 135.9±24.5 | <.001 |
| Heart rate, bpm | 75.08±18.0 | 81.9±21.2 | 78.1±20.0 | 74.0±17.2 | .005 |
| Creatinine, mg/dL | 1.03±0.4 | 1.3±0.7 | 1.4±1.0 | 1.2±0,8 | <.001 |
| Hemoglobine, g/dL | 14.0±2,0 | 13.9±2.3 | 14.0±1.7 | 14.0±3.0 | .826 |
| LDL-C, mg/dL | 110±40 | 105±40 | 113±39 | 110±40 | .042 |
| LVEF, % | 57.0±10.0 | 34.2±7.4 | 45.1±1.3 | 60.6±5.3 | <.001 |
| GRACE | 133.9±29.9 | 149.7±28.9 | 144.3±29.1 | 131.2±29.3 | <.001 |
| STEMI | 1966 (38.8) | 147 (50.7) | 299 (55.4) | 1520 (35.9) | <.001 |
| Aspirin at discharge | 4592 (90.6) | 255 (88.1) | 497 (92.1) | 3840 (90.7) | .097 |
| Clopidogrel at discharge | 3633 (71.7) | 208 (71.7) | 407 (75.5) | 3018 (71.3) | .250 |
| Beta-blockers at discharge | 3716 (73.3) | 216 (74.7) | 400 (74.1) | 3100 (73.2) | .744 |
| ACEIs-ARBs at discharge | 3210 (63.3) | 218 (75.4) | 382 (70.8) | 2610 (61.7) | <.001 |
| Statins at discharge | 4349 (85.8) | 239 (82.6) | 464 (86.0) | 3646 (86.1) | .106 |
| Mineralocorticoid receptor antagonists at discharge | 139 (2.7) | 68 (23.6) | 27 (5.0) | 44 (1.0) | <.001 |
| Diuretics at discharge | 604 (11.9) | 84 (29.1) | 80 (13.7) | 440 (10.4) | <.001 |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CABG, coronary artery bypass graft; CAD, coronary artery disease; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; LDL-C, low-density lipoprotein colesterol; LVEF; left ventricular ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as No. (%) or mean±standard deviation.
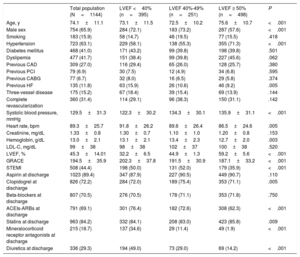
The remaining 1144 patients developed HF (Killip ≥ 2) during hospitalization: 395 (34.6%) with LVEF <40%, 251 (21.9%) with LVEF 40% to 49%, and 498 (43.5%) with LVEF ≥ 50%. Their characteristics are described in Table 2. The main differences between LVEF groups were found in age, sex, and history of hypertension, previous HF, systolic blood pressure, heart rate, hemoglobin, STEMI presentation and treatment with ACEIs, mineralocorticoid receptor antagonists, and diuretics.
Characteristics of Patients With Acute Coronary Syndrome and Heart Failure as a Function of Left Ventricular Ejection Fraction
| Total population (N=1144) | LVEF <40% (n=395) | LVEF 40%-49% (n=251) | LVEF ≥ 50% (n=498) | P | |
|---|---|---|---|---|---|
| Age, y | 74.1±11.1 | 73.1±11.5 | 72.5±10.2 | 75.6±10.7 | <.001 |
| Male sex | 754 (65.9) | 284 (72.1) | 183 (73.2) | 287 (57.6) | <.001 |
| Smoking | 183 (15.9) | 58 (14.7) | 48 (19.5) | 77 (15.5) | .418 |
| Hypertension | 723 (63.1) | 229 (58.1) | 138 (55.3) | 355 (71.3) | <.001 |
| Diabetes mellitus | 468 (41.0) | 171 (43.2) | 99 (39.8) | 198 (39.8) | .501 |
| Dyslipemia | 477 (41.7) | 151 (38.4) | 99 (39.8) | 227 (45.6) | .062 |
| Previous CAD | 309 (27.0) | 116 (29.4) | 65 (26.0) | 128 (25.7) | .380 |
| Previous PCI | 79 (6.9) | 30 (7.5) | 12 (4.9) | 34 (6.8) | .595 |
| Previous CABG | 77 (6.7) | 32 (8.0) | 16 (6.5) | 29 (5.8) | .374 |
| Previous HF | 135 (11.8) | 63 (15.9) | 26 (10.6) | 46 (9.2) | .005 |
| Three-vessel disease | 175 (15.2) | 67 (18.4) | 39 (15.4) | 69 (13.9) | .144 |
| Complete revascularization | 360 (31.4) | 114 (29.1) | 96 (38.3) | 150 (31.1) | .142 |
| Systolic blood pressure, mmHg | 129.5±31.3 | 122.3±30.2 | 134.3±30.1 | 135.9±31.1 | <.001 |
| Heart rate, bpm | 89.3±25.7 | 91.8±26.2 | 89.6±26.4 | 86.5±24.6 | .005 |
| Creatinine, mg/dL | 1.33±0.8 | 1.30±0.7 | 1.10±1.0 | 1.20±0.8 | .153 |
| Hemoglobin, g/dL | 13.0±2.1 | 13.1±2.1 | 13.4±2.3 | 12.7±2.0 | .003 |
| LDL-C, mg/dL | 99±38 | 98±38 | 102±37 | 100±38 | .520 |
| LVEF, % | 45.3±14.01 | 32.2±6.5 | 44.9±1.3 | 59.2±5.6 | <.001 |
| GRACE | 194.5±35.9 | 202.3±37.8 | 191.5±30.9 | 187.1±33.2 | <.001 |
| STEMI | 508 (44.4) | 198 (50.0) | 131 (52.0) | 179 (35.9) | <.001 |
| Aspirin at discharge | 1023 (89.4) | 347 (87.9) | 227 (90.5) | 449 (90.7) | .110 |
| Clopidogrel at discharge | 826 (72.2) | 284 (72.0) | 189 (75.4) | 353 (71.1) | .005 |
| Beta-blockers at discharge | 807 (70.5) | 276 (70.5) | 178 (71.1) | 353 (71.8) | .750 |
| ACEIs-ARBs at discharge | 791 (69.1) | 301 (76.4) | 182 (72.6) | 308 (62.3) | <.001 |
| Statins at discharge | 963 (84.2) | 332 (84.1) | 208 (83.0) | 423 (85.8) | .009 |
| Mineralocorticoid receptor antagonists at discharge | 215 (18.7) | 137 (34.6) | 29 (11.4) | 49 (1.9) | <.001 |
| Diuretics at discharge | 336 (29.3) | 194 (49.0) | 73 (29.0) | 69 (14.2) | <.001 |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CABG, coronary artery bypass graft; CAD, coronary artery disease; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; LDL-C, low-density lipoprotein colesterol; LVEF, lef ventricular ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as No. (%) or mean±standard deviation.
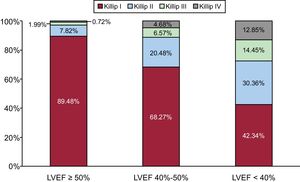
The incidence of HF was increasingly higher in each category of LVEF (Figure 2). In-hospital mortality was 4.0% (95%CI, 3.4-4.5) and was increasingly higher in each LEVF category: 1.75% (LVEF ≥ 50%), 5.44% (LVEF 40%-49%), and 17.60% (LVEF <40%) (P <.01), as well as in patients who developed HF compared with those who did not: 22.5% vs 1.4% (P <.01).
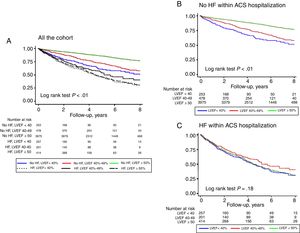
Postdischarge PrognosisPostdischarge follow-up was available in 91.8% of the patients with a median of 4.4 years [interquartile range, 2.2-6.5]. All-cause mortality was 22.5% (95%CI, 21.5-23.5) at was much higher in patients with HF during the ACS hospitalization (50.4% vs 17.6%; P <.001). As shown in Figure 3A, in patients without HF during the ACS hospitalization, the lowest rate was noted in patients LVEF ≥ 50% (15.4%), followed by those with LVEF 40% to 49% (25.4%) and LVEF <40% (29.4%); in contrast, no differences were observed in patients who developed HF during the ACS hospitalization (Figure 3B).
We performed a landmark analysis with the assessment of 1-year mortality and the results for all-cause mortality were the same ().
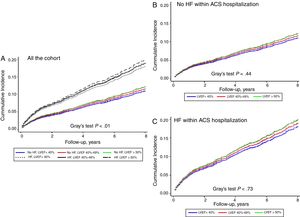
The incidence of HF after hospital discharge was 13.3% (95%CI, 12.5-14.1) and was higher in patients with HF during the ACS hospitalization (31.5% vs 10.0%; P <.001). As shown in Figure 4A, among patients without HF during the ACS hospitalization, the highest rate was found in those with LVEF <40% (18.2%) or LVEF 40% to 49% (17.2%), and was much lower in patients with LVEF ≥ 50% (8.3%). Nonetheless, no differences were observed according to LVEF among patients with HF during the ACS hospitalization (Figure 4B).
Mortality and Heart Failure PredictorsMultivariate analysis of all-cause mortality and HF was adjusted by age, sex, diabetes mellitus, previous coronary artery disease, previous HF, complete revascularization, treatment with diuretics, ACEIs, and beta-blockers, and LVEF.
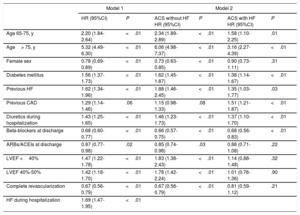
Heart failure during the hospitalization, LVEF <40%, and LVEF 40% to 49% were associated with all-cause mortality and postdischarge HF in a first analysis (Table 3), and a positive interaction was found between HF at admission and LVEF; therefore, the final model was designed with these interactions. The discriminative (Harrell's C-statistic, 0.79; 95%CI, 0.78-0.80) and calibration (Gronnesby and Borgan test P=.73) ability of the survival models were high. Higher LVEF, as well as complete revascularization and female sex, was associated with lower mortality only in patients without HF during the ACS hospitalization. The effect of age on long-term mortality was lower in patients who had HF during hospitalization (P=.01).
Results of the Multivariate Analysis Assessing Independent Predictors of All-cause Mortality
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| HR (95%CI) | P | ACS without HF HR (95%CI) | P | ACS with HF HR (95%CI) | P | |
| Age 65-75, y | 2.20 (1.84-2.64) | <.01 | 2.34 (1.89-2.89) | <.01 | 1.58 (1.10-2.25) | .01 |
| Age> 75, y | 5.32 (4.49-6.30) | <.01 | 6.06 (4.98-7.37) | <.01 | 3.16 (2.27-4.39) | <.01 |
| Female sex | 0.78 (0.69-0.89) | <.01 | 0.73 (0.63-0.85) | <.01 | 0.90 (0.73-1.11) | .31 |
| Diabetes mellitus | 1.56 (1.37-1.73) | <.01 | 1.62 (1.45-1.87) | <.01 | 1.38 (1.14-1.67) | <.01 |
| Previous HF | 1.62 (1.34-1.96) | <.01 | 1.88 (1.46-2.45) | <.01 | 1.35 (1.03-1.77) | .03 |
| Previous CAD | 1.29 (1.14-1.46) | .06 | 1.15 (0.98-1.33) | .08 | 1.51 (1.21-1.87) | <.01 |
| Diuretics during hospitalization | 1.43 (1.25-1.65) | <.01 | 1.46 (1.23-1.73) | <.01 | 1.37 (1.10-1.70) | <.01 |
| Beta-blockers at discharge | 0.68 (0.60-0.77) | <.01 | 0.66 (0.57-0.75) | <.01 | 0.68 (0.56-0.83) | <.01 |
| ARBs/ACEIs at discharge | 0.87 (0.77-0.98) | .02 | 0.85 (0.74-0.98) | .03 | 0.88 (0.71-1.08) | .22 |
| LVEF <40% | 1.47 (1.22-1.78) | <.01 | 1.83 (1.38-2.43) | <.01 | 1.14 (0.88-1.48) | .32 |
| LVEF 40%-50% | 1.42 (1.18-1.70) | <.01 | 1.78 (1.42-2.24) | <.01 | 1.01 (0.76-1.36) | .90 |
| Complete revascularization | 0.67 (0.56-0.79) | <.01 | 0.67 (0.56-0.79) | <.01 | 0.81 (0.59-1.12) | .21 |
| HF during hospitalization | 1.69 (1.47-1.95) | <.01 | ||||
95%CI, 95% confidence interval; ACEIs, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; ARBs, angiotensin receptor blockers; CAD, coronary artery disease; HF, heart failure; HR, hazard ratio; LVEF; left ventricular ejection fraction.
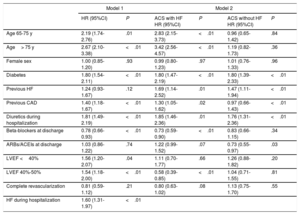
Independent predictors of postdischarge HF are presented in Table 4. Left ventricular ejection fraction 40% to 49% was independently associated with lower postdischarge HF only in patients who did not develope HF during hospitalization. The discriminative (Harrell's C-statistic, 0.76; 95%CI, 0.72-0.81) and calibration (Gronnesby and Borgan test P=.73) ability of competing risk regression models were adequate for their intended purpose. As an intern sensitivity analysis, we investigated the possible changes in clinical features of patients throughout the long inclusion period by dividing the cohort into 3 time intervals (2004-2006, 2007-2010, and 2011-2014). No differences were found in age or sex and only a slight increase in the prevalence of hypertension was noted. The percentage of STEMI patients increased significantly during the inclusion period but the mean GRACE score did not. The prevalence of LVEF <40% (15.7%, 15.8%, 18.6%; P=.09) or HF during hospitalization (18.3%, 19.3%, 17.6%; P=.337) did not vary between the 3 time periods.
Results of the Multivariate Analysis Assessing Independent Predictors of Postdischarge Heart Failure
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| HR (95%CI) | P | ACS with HF HR (95%CI) | P | ACS without HF HR (95%CI) | P | |
| Age 65-75 y | 2.19 (1.74-2.76) | .01 | 2.83 (2.15-3.73) | <.01 | 0.96 (0.65-1.42) | .84 |
| Age> 75 y | 2.67 (2.10-3.38) | <.01 | 3.42 (2.56-4.57) | <.01 | 1.19 (0.82-1.73) | .36 |
| Female sex | 1.00 (0.85-1.20) | .93 | 0.99 (0.80-1.23) | .97 | 1.01 (0.76-1.33) | .96 |
| Diabetes | 1.80 (1.54-2.11) | <.01 | 1.80 (1.47-2.19) | <.01 | 1.80 (1.39-2.33) | <.01 |
| Previous HF | 1.24 (0.93-1.67) | .12 | 1.69 (1.14-2.52) | .01 | 1.47 (1.11-1.94) | <.01 |
| Previous CAD | 1.40 (1.18-1.67) | <.01 | 1.30 (1.05-1.62) | .02 | 0.97 (0.66-1.43) | <.01 |
| Diuretics during hospitalization | 1.81 (1.49-2.19) | <.01 | 1.85 (1.46-2.36) | .01 | 1.76 (1.31-2.36) | <.01 |
| Beta-blockers at discharge | 0.78 (0.66-0.93) | <.01 | 0.73 (0.59-0.90) | <.01 | 0.83 (0.66-1.15) | .34 |
| ARBs/ACEIs at discharge | 1.03 (0.86-1.22) | .74 | 1.22 (0.99-1.52) | .07 | 0.73 (0.55-0.97) | .03 |
| LVEF <40% | 1.56 (1.20-2.07) | .04 | 1.11 (0.70-1.77) | .66 | 1.26 (0.88-1.82) | .20 |
| LVEF 40%-50% | 1.54 (1.18-2.00) | <.01 | 0.58 (0.39-0.85) | <.01 | 1.04 (0.71-1.55) | .81 |
| Complete revascularization | 0.81 (0.59-1.12) | .21 | 0.80 (0.63-1.02) | .08 | 1.13 (0.75-1.70) | .55 |
| HF during hospitalization | 1.60 (1.31-1.97) | <.01 | ||||
95%CI, 95% confidence interval; ACEIs, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; ARBs, angiotensin receptor blockers; CAD, coronary artery disease; HF, heart failure; HR, hazard ratio; LVEF; left ventricular ejection fraction.
In our study of patients with ACS from 2 Spanish hospitals, we found that LVEF in patients with HF was not related to long-term prognosis. In contrast, in patients without HF during hospitalization, LVEF was a strong predictor of prognosis. We suggest that the management of ACS complicated with HF is still challenging, irrespective of the LVEF category. Our findings confirm and emphasize that a clinical diagnosis of HF in patients with ACS is a sign of an ominous prognosis even in patients with normal LVEF, even nowadays, when ACS patients are provided with highly effective invasive and noninvasive treatment strategies.
Independently of the presence of HF, patients with LVEF <40% were predominantly men, with previous coronary artery disease and revascularization and more comorbidities, as previously described19 (Table 1 and Table 2). This group of patients represented the majority in the HF group (34.6%) compared with the non-HF group, in which LVEF ≥ 50% was the predominant form (83.4%).
The midrange LVEF group represented a small proportion of patients with ACS and these patients had an intermediate demographic and clinical profile with many intermediate features between the reduced and preserved LVEF groups (Table 1 and Table 2). These results are similar to those observed in acute decompensated and chronic HF patients.14,20 The group of patients with ACS and HF and LVEF ≥ 50% had a similar profile to those with HF with preserved ejection fraction, being older and more frequently female and hypertensive compared with the other groups.21
Similar to previous investigations,12,19 we observed that during follow-up HF patients had higher mortality and HF readmissions compared with non-HF patients.
It has been over 40 years since Killip first described the importance of the clinical signs of heart dysfunction and failure after acute myocardial infarction as mortality risk assessment.22 Since then, numerous studies have identified HF in the setting of myocardial infarction as an important predictor of prognosis.22,23 Nowadays, in an era of highly effective treatment strategies, there is a marked decrease in the incidence of HF complicating ACS. However, HF continues to worsen the early-, intermediate-, and long-term prognostic risk after ACS23 and little information is avaliable about the management of this syndrome in the current interventional era.
The prognostic value of LVEF in patients with ACS and HF is not well described. In the VALIANT-registry, HF with systolic dysfunction was associated with more complications, longer hospitalizations, and higher long-term mortality.7 In 1988, in the noninvasive era of ACS management, Nicod et al.,24 suggested that the prognostic value of HF complicating ACS did not depend on LVEF.
The recent Acute Coronary Syndrome Israeli Survey found that LVEF was a powerful predictor of mortality 1 year after ACS, independently of the presence of HF,25 but the SWEDEHEART registry has shown that patients with ACS and HF with preserved LVEF had only a slightly better long-term prognosis than patients with HF with reduced LVEF,12 who had a much higher mortality rate than patients discharged without signs of HF, regardless of LVEF.12
In our study, we found similar results; patients who developed HF during hospitalization had higher rates of mortality and HF readmission during follow-up. However, this poor prognosis did not depend on LVEF (Figure 3 and Figure 4), suggesting that, in ACS patients, once HF syndrome has become established during hospital stay, the long-term prognosis was unrelated to LVEF and all these patients should be managed as very high risk. Regardless of these findings, several registries have observed that there is a chance to improve the management of these high risk population.4,23
The role of complete revascularization in ACS patients with and without HF for mortality and morbidity (HF worsening or development) risk reduction is controversial.26 Current guidelines recommend a culprit-lesion revascularization strategy and the treatment of the chronic lesions according to the presence of ischemia/angina.13 However, the results of the recently published STICH subanalysis question the usefulness of systematic revascularization of patients with angina and reduced LVEF.27 In our centers, we carry out this strategy, which could influence the proportion of patients with complete revascularization.
In historical and contemporary cohorts of ACS, LVEF has been described as an important predictor of clinical outcomes after acute myocardial infarction.19,28,29 The impairment of LVEF after an ACS may be due to irreversible myocardial damage and the remodeling process that causes progressive dilatation and deterioration in contractile function leading to HF, mortality, and lethal arrhythmias.30
Our findings showed that LVEF during hospital admission was a strong mortality determinant in non-HF patients. The group with LVEF <40% had a much worse prognosis compared with the other 2 groups (LVEF 40%-49%, and ≥ 50%). In addition, in non-HF patients LVEF <40% was associated with HF after discharge; the midrange ejection fraction group showed a tendency of better outcome in terms of HF readmission, probably because they were more similar to patients with preserved LVEF.14
Similar to data highlighted by other authors in non-HF patients,19,31,32 we found that age, female sex, diabetes mellitus, previous coronary artery disease, previous HF, complete revascularization, and treatment with diuretics, ACEIs and beta-blockers were predictors of outcome (Table 3 and Table 4).
In our study, women were more likely to have preserved systolic function in both groups (HF and non-HF) and tended to have more comorbidities. The mechanism behind this remains unknown, although while some publications have suggested possible intrinsic sex-based differences in the cardiac remodeling process after ACS.12,23
Strenghts and LimitationsThis study has several strengths; it represents a contemporary cohort with a large sample and statistical power so the results may be generalizable to real-world clinical practice. We included a large number of patients with a very long follow-up. We examined the different prognosis of patients with ACS and LVEF <40%, 40% to 49%, and ≥ 50% and also analyzed the long-term prognostic factors depending on HF development.
The study also has some limitations. First, it is an observational and retrospective analysis and we could not measure LVEF in 12.2% of the study population; previous studies have reported that missing echocardiographic data is not uncommon in clinical practice.33 In addition, 8.2% of patients were lost to follow-up. We used the classification proposed by the ESC-HF guidelines in the ACS setting, which could be a study limitation. As in any observational study, we cannot rule out the effect of residual confounding due to unmeasured variables. In addition, there may be appropriate contraindications to adjunctive pharmacotherapy or invasive angiography that were not collected or known to us. We used the Simpson method to estimate LVEF according to international recommendations with its inherent limitations. We had no data on any biomarker with utility in HF, such as B-type natriuretic peptide, midregional proatrial natriuretic peptide, soluble ST2, or galectin-3. Our study began in 2004, which explains why the use of beta-blockers and ACEIs was lower than estimated. Over the years, the use of these drugs has increased progressively in both institutions. We did not identify treatment adherence or changes in LVEF during follow-up. In addition, the percentage of ACEIs, beta-blockers and mineralocorticoid antagonist that was maintained during admission is unknown. Finally, revascularization and complete revascularization rates were not too high, which merely reflects daily clinical practice in 2 centers with available catheterization laboratories, as previously described.
CONCLUSIONSIn a large cohort of patients with ACS, we showed that LVEF in the subgroup complicated with HF was not related to long-term prognosis. In the subgroup of patients without clinical HF syndrome during hospital stay, LVEF was a strong prognostic predictor. The poor prognosis of patients with ACS and HF suggest that therapeutic efforts should be focused on this group of patients regardless of LVEF.
FUNDINGThe present study was supported by Complejo Hospitalario Universitario de Santiago de Compostela (Santiago de Compostela, Spain) and was cofunded by the Instituto de Salud Carlos III-Subdirección General de Evaluación y Fomento de la Investigación y el Fondo Europeo de Desarrollo Regional.
CONFLICTS OF INTERESTNone declared.
- –
Contemporary data are scarce on the incidence and prognosis of HF and the influence of LVEF and the new ESC-HF classification based on this parameter in an ACS setting.
- –
In ACS patients, we found that the development of HF during thospital stay was associated with a worse long-term prognosis compared with patients without HF, irrespective of LVEF. This parameter is a strong predictor of prognosis only in patients without HF.
- –
The poor prognosis of patients with ACS and HF suggests that therapeutic efforts should focus on this group, regardless of LVEF.