Apical hypertrophic cardiomyopathy (ApHCM) accounts for just 1%-10% of all HCM cases in the Western world, although it is much more common in Eastern countries (up to 40%).1 ApHCM is characterized by hypertrophy at the apex of the left ventricle and possibly also midventricular hypertrophy or hypertrophy at another level. This increased thickness reduces the size of the ventricular cavity, resulting in diminished ventricular volume and increased end-diastolic pressure.2 Because of the morphologic changes characterizing ApHCM, the predominant clinical manifestation is dyspnea due to heart failure with preserved ejection fraction, although other complications such as sudden cardiac death and stroke have been reported.3 While HCM with left ventricular outflow tract obstruction can be treated with various drugs or surgery, the treatment of diastolic dysfunction in ApHCM is much more difficult and few alternatives exist. Heart transplant is the only option for patients with a high functional class.
In 2010, Schaff et al.4 described the use of apical myectomy, a procedure involving the removal of excess ventricular muscle through the apex of the left ventricle, to treat ApHCM. Our group started such a program 2016 and our hospital is currently the only institution in Spain with an active program. In this article, we describe our experience with the use of apical myectomy in the treatment of ApHCM.
Between January 2016 and August 2020, 6 patients underwent apical myectomy for ApHCM at our hospital. Their baseline characteristics and clinical and echocardiographic findings are shown in table 1. Five patients had subaortic and midventricular obstruction, and none had apical aneurysm. They were all in New York Heart Association (NYHA) class III-IV despite medical treatment; 2 were being evaluated for a heart transplant. Apical myectomy was elective in all cases. After establishment of cardiopulmonary bypass and clamping of the aorta, an apical left ventriculotomy was performed with wide excision of the hypertrophic myocardium of the interventricular septum, the free wall, and the base of the papillary muscles. In 5 cases, an aortic incision was also made to perform subaortic septal myectomy. One of the patients also had myxoid degeneration and underwent mitral valve repair with annuloplasty and chordal implantation. The median hospital stay was 9 days (interquartile range [IQR], 8-12 days].
Baseline patient characteristics and clinical and echocardiographic findings
| Variable | |
|---|---|
| Age, y | 61 [40-68] |
| Sex | |
| Male | 1 (16.7) |
| Female | 5 (83.3) |
| Dyspnea (NYHA III-IV) | 6 (100) |
| Syncope | 2 (33.3) |
| Angina | 3 (50) |
| Diabetes | 2 (33.3) |
| ICD | 2 (33.3) |
| Dyslipidemia | 1 (16.7) |
| Hypertension | 2 (33.3) |
| Stroke | 1 (16.7) |
| Ventricular tachycardia | 0 |
| Atrial fibrillation | 1 (16.7) |
| Pacemaker | 0 |
| Variable | Preoperative | Postoperative |
|---|---|---|
| Dyspnea (NYHA III-IV) | 6 (100) | 1 (16.7) |
| NT-proBNP, pg/dL | 2.122 [734-2418] | 1.040 [732-1040] |
| Beta-blocker | 5 (83.3) | 5 (83.3) |
| Disopyramide | 4 (66.7) | 0 |
| ACE inhibitor | 1 (16.7) | 1 (16.7) |
| Calcium channel blocker | 2 (33.3) | 0 |
| Diuretics | 5 (83.3) | 3 (50) |
| Echocardiogram | ||
| LVEF, % | 68 [61-78] | 55 [44-66] |
| LVEDV, mm | 60.5 [44.8-70.7] | 98.5 [76.1-141.0] |
| LVESV, mm | 21.1 [15.4-24.9] | 42.2 [28.0-66.7] |
| Basal septal thickness | 18.2 [12.2-24.5] | 13 [8.1-19] |
| Maximum midventricular thickness | 26 [22.5-28.2] | 16.5 [13.7-19.2] |
| Midventricular obstruction | 4 (66.7) | 0 |
| Mitral insufficiency >2 | 1 (16.7) | 1 (16.7) |
| SAM | 2 (33.3) | 0 |
ACE, angiotensin-converting enzyme; ICD, implantable cardioverter-defibrillator; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; NT-proBNP, prohormone brain-type natriuretic peptide; SAM, systolic anterior motion of the mitral valve.
Values are expressed as No. (%) or median [interquartile range].
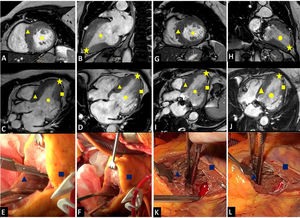
Functional class (NYHA I-II) significantly improved in 5 patients after a median follow-up of 18 months [IQR, 6-24 months]; the sixth patient remained in NYHA III. The preoperative and postoperative clinical and echocardiographic findings are shown in table 1. The echocardiographic study, performed after 6 months in 5 patients and after 1 month in 1 patient, showed a significant increase in ventricular volume in all patients. Images of the surgical field and the ventricular volumes before apical myectomy and during follow-up are shown in figure 1. One patient who had achieved a postoperative improvement to NYHA class I-II developed severe mitral insufficiency due to chordal rupture 14 months after surgery and experienced functional deterioration. She underwent MitraClip placement.
Left ventricular diastolic volumes before surgery and during follow-up and images of the surgical field. Preoperative magnetic resonance images showing a 2-chamber short-axis view (A), a 2-chamber long-axis view (B), a 3-chamber long-axis view (C), and a 4-chamber long-axis view (D). E, ventriculotomy. F, surgical field before myectomy. Follow-up magnetic resonance images showing a 2-chamber short-axis view (G), a 2-chamber long-axis view (H), a 3-chamber long-axis view (I), and a 4-chamber long-axis view (J). K and L, surgical field after myectomy. Circle, left ventricular end-diastolic volume; triangle, interventricular septum; square, left ventricle free wall; star, left ventricular apex.
ApHCM is very difficult to treat in patients who do not respond adequately to medical treatment and is associated with an increased risk of death.3
Apical myectomy has been proposed as an alternative treatment for selected patients with ApHCM. The Mayo Clinic group recently reported outcomes for 113 patients with ApHCM and advanced heart failure treated with apical myectomy between September 1993 and March 2017.5 In-hospital mortality was 4%, functional class improved in 76%, and heart transplant was required during follow-up in 3%. The respective 1-, 5-, and 10-year survival rates were 96%, 87%, and 74%, which are higher than rates reported for patients with ApHCM listed for a heart transplant in the United States. In Spain, 115 patients with HCM underwent a heart transplant between 2015 and 2019 (7.5% of all heart transplants performed), and the annual trend was toward an increase in cases (unpublished data courtesy of Francisco González-Vílchez from the Spanish National Heart Transplant Registry). Although it is not known how many of these patients had ApHCM, it is likely that some of them may have benefited from apical myectomy.
Although our initial experience is limited, the outcomes at our hospital are similar to those reported by the Mayo Clinic,4,5 indicating that with adequate training and correct diagnosis and perioperative treatment, apical myectomy can be successfully performed in specialized hospitals.
Apical myectomy is a safe and effective technique for the treatment of ApHCM and could offer an alternative to heart transplant in patients with advanced heart failure.