Hyperglycemia is common in survivors of out-of-hospital cardiac arrest (OHCA). It is associated with increased mortality and worse neurological outcomes. Current guidelines advise its treatment,1 but the optimal blood glucose level for this population has not been evaluated in randomized clinical trials.
To determine whether in-hospital glycemic control is associated with neurological outcome at 6 months, we performed an ambispective observational study of a cohort of OHCA survivors admitted to the acute cardiac care unit of our center between May 2019-April 2021. The study was approved by the ethics committee and all patients/relatives signed the informed consent form. The inclusion criterion was being admitted for an OHCA and remaining comatose after return of spontaneous circulation (ROSC). The exclusion criteria were refusal to provide informed consent and being lost to follow-up. As part of in-hospital standard management, intravenous or subcutaneous basal-bolus insulin regime treatment protocols were applied for all patients as per treating physician discretion. Point of care glycemia measurements were performed before the morning, afternoon, evening meals, and at midnight during the basal-bolus insulin regime, or hourly during intravenous treatment. The insulin dose was modified, with the goal of maintaining blood glucose levels between 100-180mg/dL over the entire hospitalization. For each patient, the values retrieved were: first blood glucose, mean blood glucose during hospitalization (BGOH), blood glucose in the morning, afternoon, evening and night, maximum blood glucose, and percentage of blood glucose values within the 100-180 mg/dL target. Insulin doses could not be retrieved from the system properly.
Evaluation of neurological outcome was structured, multimodal and multidisciplinary. Withdrawal of life-sustaining therapy was decided in consensus with the entire the team, concluding the patient had poor neurological outcome. Neurological outcome at 6 months was evaluated according to the cerebral performance category (CPC) score. Patients were divided into 2 groups: “good” (CPC 1–2) or “poor” (CPC 3–5) neurological outcome. Patients who died were included in this last group.
Univariate analysis was performed between the 2 groups. A stepwise logistic regression model was performed to determine covariates independently associated with neurological outcome.
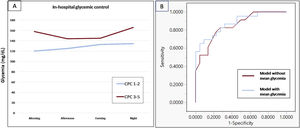
After an initial screening log of 50 patients, 45 patients were included in the final analysis (3 were lost to follow-up and 2 did not sign the informed consent form). Median age was 60.1 [interquartile range, 50.9-70.6] years, most were male (86.7%), 22 (48.9%) patients had good neurological outcome (CPC 1-2) and mortality at 6 months was 46.7% (most deaths—85.7%—occurred during hospitalization and were the consequence of neurological injury and/or withdrawal of life-sustaining therapy). Demographic and clinical characteristics were similar between the 2 groups: 76% had a first shockable rhythm and an acute coronary syndrome was the cause in 58%. All patients required mechanical ventilation and were treated with active targeted temperature management in agreement with current guidelines.1 Most of them (89%) received vasopressor/inotrope support. Blood glucose, lactate, APACHE-II, and SOFA scores were higher in patients with CPC 3-5 (table 1). A total of 3190 blood glucose determinations were performed during hospitalization. Patients with good neurological outcome had lower BGOH mg/dL (130 [124-138] vs 166 [131-187], P <.01), lower glycemia at all time slots (figure 1), and a higher percentage of glycemic controls within the 100-180mg/dL target (83.8% vs 64.8%; P <.01). Moreover, maximum glycemia was lower in patients with good neurological outcome (218 [180-259] vs 278 [232-420]; P <.01). As expected, BGOH was lower in nondiabetic individuals (132 [124-138] vs 185 [130-217]; P <.01). However, we found no interaction between glycemic control variables and diabetes status in the association with neurological outcome. In the logistic regression analysis, only BGOH (odds ratio, 1.03; 95% confidence interval, 1.006-1.07) and APACHE-II (odds ratio, 1.34; 95% confidence interval, 1.10-1.64) were independently associated with neurological outcome (area under the curve of the model 0.879, P <.01).
Baseline demographic and clinical characteristics
| CPC 1-2 (n=22) | CPC 3-5 (n=23) | P | |
|---|---|---|---|
| Age, y | 57.3 [49.5-62.7] | 65.2 [54.9-79.9] | .02 |
| Male sex | 19 (42.2) | 20 (44.4) | .95 |
| Female sex | 3 (6.7) | 3 (6.7) | |
| ACCU length of stay, d | 8 [6-11.5] | 8 [4-11] | .43 |
| Hospital length of stay, d | 32 [20.5-50] | 44 [23-86] | .20 |
| Time from admission to death, d* | - | 36 [22-54.25] | - |
| Glycemia at admission, mg/dL | 202.1 (126.4-277.8) | 285.9 (167.1-404.7) | <.01 |
| HbA1c percentage | 5.5 (5.2-5-7) | 5.6 (5.5-6.1) | .09 |
| Troponin maximum ng/L | 10 049.5 (3024.8-50 520.5) | 22 187 (4013-82 896) | .38 |
| Lactate maximum mg/dL | 45.5 (30.8-68.5) | 70 (39-92) | .03 |
| BMI kg/m2 | 26.5 (24.7-28.8) | 26.3 (24.7-28.3) | .96 |
| Hypertension | 8 (36.4) | 14 (60.9) | .11 |
| Dyslipidemia | 9 (40.9) | 14 (60.9) | .18 |
| Diabetes | 3 (13.6) | 6 (26.1) | .29 |
| COPD | 2 (9.1) | 6 (12.8) | .10 |
| Stroke | 0 | 0 | - |
| CKD | 1 (4.6) | 2 (8.7) | .58 |
| CLD | 2 (9.1) | 0 | .14 |
| Neoplasia | 1 (4.6) | 1 (4.4) | .97 |
| PVD | 0 | 4 (17.4) | .04 |
| Tobacco | 11 (50) | 6 (26.9) | .10 |
| Ethanol | 3 (13.6) | 3 (13) | .95 |
| Chronic coronary syndrome | 3 (13.6) | 3 (13) | .95 |
| Other cardiomyopathy | 4 (18.2) | 6 (26.1) | .52 |
| NYHA 1-2 | 21 (95.5) | 22 (95.6) | .91 |
| NYHA 3-4 | 1 (4.5) | 1 (4.4) | |
| Shockable rhythm | 18 (81.8) | 16 (69.6) | .34 |
| ROSC, minutes | 19.5 [11-37.5] | 30 [24-38] | .06 |
| Prehospital adrenaline dosage, mg | 1 (0.4-1.6) | 2 (1.5-2.5) | .49 |
| Acute coronary syndrome | 12 (54) | 14 (60) | .67 |
| Revascularization | 14 (64) | 14 (61) | .17 |
| Ejection fraction, % | 45 [33.8-55] | 45 [35-55] | .55 |
| SOFA score | 9 [6.5-11] | 11 [8-12] | .08 |
| APACHE-II score | 19 [17-22.3] | 26 [22-30] | <.01 |
ACCU, acute cardiac care unit; APACHE-II score; acute physiology and chronic health disease classification system II; BMI, body mass index; CKD, chronic kidney disease; CLD, chronic liver disease, COPD; chronic obstructive pulmonary disease; CPC, cerebral performance category; HbA1c, glycosylated hemoglobin; NYHA, New York Heart Association functional classification;PVD, peripheral vascular disease; ROSC, time to return of spontaneous circulation; SD, standard deviation; SOFA, sequential organ failure assessment score.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
A: in-hospital glycemic control based on neurological outcome at 6 months. B: comparison of the regression model receiving operator curves for neurological outcome. Adding mean glycemia in the model improved the area under the curve from 0.848 (red curve) to 0.879 (blue curve). CPC, cerebral performance category.
In this observational study of comatose survivors of OHCA, lower average blood glucose values during hospitalization, irrespective of diabetic status, were associated with better neurological outcome at 6 months. Previous studies found consistent results. A multicenter analysis2 determined that in survivors of OHCA, glycemia between 70-140mg/dL was associated with lower in-hospital mortality. Like our results, these data suggest that OHCA survivors could benefit from lower glycemic targets during hospitalization. Nevertheless, the last ILCOR guidelines recommend a glycemic target of 140-180mg/dL, and avoidance of hypoglycemia1 since tight glycemic control and hypoglycemia have also been associated with poor neurological outcome.3 Hence a U-shaped relationship between blood glucose and neurological outcome has been suggested in OHCA patients, although the optimal range has not yet been defined.
Patients with diabetes included in this study had higher glycemia and lower percentage of blood glucose measurements within the prespecified target during hospitalization, but diabetic status was not independently associated with neurological outcome. Some studies have found a worse neurological outcome in individuals with diabetes after OHCA.4 In our cohort, patients with diabetes (20%) had good glycemic control (average HbA1c 6.4%), which could explain their similar outcome.
The performance of APACHE-II to predict neurological outcome after OHCA is controversial, which might be related to the time when it is measured.5 In the present study, APACHE-II was measured within 24hours after ROSC and had a very strong association with neurological outcome, and a good discriminatory ability: an APACHE-II value of 23 had 73.9% sensitivity and 77.3% specificity for predicting poor neurological outcome.
The main limitation of this study is its observational nature, which precludes many potential confounding factors and only allows finding statistical associations but not causality. In addition, glycemic determinations were performed as part of usual care. However, the study is hypothesis generating and could help in the design of future work in OHCA patients investigating the optimal BGOH target to improve outcomes.
In conclusion, in survivors of OHCA, lower BGOH is independently associated with better neurological outcome at 6 months, but the optimal blood glucose level for this population is still to be defined. The APACHE-II score is also strongly associated with and has good predictive ability for neurological outcome after ROSC.
FUNDINGNot funded.
AUTHORS’ CONTRIBUTIONSJ.C. Valerio-Rojas: drafting the work, acquisition, analysis, and interpretation of data. M. Izquierdo: patient inclusion, acquisition, analysis, and interpretation of data, revising the manuscript critically for important intellectual content. O. de Diego: patient inclusion, acquisition, analysis, and interpretation of data, revising the manuscript critically for important intellectual content. E. Ortega: drafting the work, acquisition, analysis, and interpretation of data. I. Conget: analysis and interpretation of data, revising the manuscript critically for important intellectual content. R. Andrea: design and planning of the work, patient inclusion, acquisition, analysis, and interpretation of data, drafting the work, manuscript submission.
CONFLICTS OF INTERESTThe authors have no conflicts of interest to disclose directly related to the matter discussed in this manuscript.