The prognosis of asymptomatic severe aortic stenosis (AS) has not been widely documented in elderly patients who are frequently frail and have comorbidities. We sought to analyze the factors that influence early mortality in geriatric patients with asymptomatic severe AS.
MethodsThis ambispective cohort study included 104 patients aged 70 years or older with asymptomatic severe AS. Epidemiological, geriatric, clinical and echocardiographic variables were collected and compared between frail and nonfrail patients. During follow-up, the time from diagnosis to mortality and the causes of death were recorded.
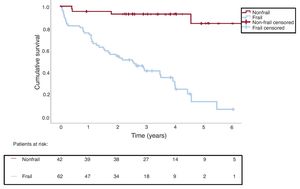
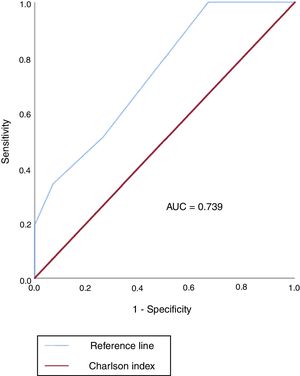
ResultsOverall, 59.6% of the patients were frail. During follow-up, 69.4% of the frail patients died, with a median time to mortality of 2.52 years (95%CI, 1.36-3.69). The overall 1-year survival rate in frail patients was 76%. On multivariate analysis, age (HR, 2.47; 95%CI, 1.00-6.12), a Charlson comorbidity index ≥ 5 (HR, 3.75; 95%CI, 1.47-9.52) and frailty (HR, 6.67; 95%CI, 1.43-9.52) were independently related to mortality. In total, 8.7% of the patients had a Charlson comorbidity index ≥ 5, and all these patients died during follow-up, with a median survival of 1.01 years (95%CI, 0.36-1.67). The area under the receiver operating characteristic curve of the Charlson index was 0.739 (95%CI, 0.646-0.832). In this population, values ≥ 5 showed high specificity (100%) but low sensitivity.
ConclusionsA high prevalence of frailty was present in geriatric patients with asymptomatic severe AS. Age, a Charlson index ≥ 5 and frailty were independent factors for mortality, conferring an unfavorable short-term prognosis.
Keywords
Evidence of the natural history of severe asymptomatic aortic stenosis (AS) in elderly individuals is lacking because the main study series have predominantly included patients younger than 70 years of age.1,2 The aged population presents special features, such as a high number of comorbidities and geriatric syndromes. In addition, the detection of early symptoms in elderly patients may be difficult, especially if their physical mobility is limited.
The presence of frailty is relatively common in patients with heart disease. This condition is a geriatric syndrome characterized by increased vulnerability to minor stressors, resulting in a decline in multiple physiologic systems.3 Frailty plays a major role in the selection of candidates for transcatheter aortic valve implantation (TAVI)4–6 and aortic valve replacement (AVR) in the geriatric population with severe AS.7–9 In this population, measures of frailty are highly predictive of poor outcomes, including death, incident disability, and hospitalization.10 In fact, in approximately 40% of patients who undergo TAVI, the procedure is futile, resulting in poor outcomes and a lack of improvement in survival and/or quality of life.11 Clinical practice guidelines recommend an evaluation to optimize the selection of patients referred for invasive treatment.12,13 However, there is a lack of consensus on the best tool to assess frailty.14 On the other hand, AS may be the cause of frailty, and there are aspects of frailty that may be reversible and susceptible to rehabilitation.15 Consequently, a clinical assessment of several geriatric and cardiological factors that influence this asymptomatic population may be vital to prevent early mortality.
Other variables related to age, such as comorbidity, functionality, cognitive status and quality of life, can influence the prognosis of these patients.16 All of these factors should be included in the comprehensive geriatric assessment. Regarding highly prevalent comorbidities in geriatric patients, some noncardiac conditions impact prognoses in the short- and long-term, irrespective of management. Therefore, the inclusion of an assessment of comorbidities along with the Charlson comorbidity index in a preoperative study17 was shown to be useful in predicting poor outcomes in patients who are candidates for invasive treatment.18
Our aim was to define the risk factors that influence early mortality in an elderly population with severe asymptomatic degenerative AS.
METHODSStudy designThis longitudinal, ambispective, cohort observational study included 104 geriatric patients aged 70 years or older who were diagnosed with severe asymptomatic AS. Patients were recruited from a specific valve disease outpatient clinic. The inclusion period was from January 2010 to January 2016, and follow-up was completed in June 2017. The exclusion criteria consisted of the concomitant presence of another moderate-severe valve disease, previous aortic valve surgery, the presence of symptoms at the time of the recruitment period or echocardiographic criteria for AVR.19 The collected biodemographic data were age, sex, weight, height, body mass index, and corporal surface. The clinical variables examined were cardiovascular risk factors such as high blood pressure, dyslipidemia, diabetes mellitus type 2, New York Heart Association classification, the presence of atrial fibrillation, and the EuroSCORE logistic surgical risk score.20 All the variables required to calculate the Charlson comorbidity index were collected.17 Our population was dichotomized according to a cutoff point ≥ 5 to determine the influence of the Charlson comorbidity index on mortality.18 Frailty was evaluated using the Fatigue, Resistance, Ambulation, Illness, and Loss of Weight (FRAIL) questionnaire conducted by the same cardiologist at the time of recruitment21,22; the patient was defined as frail when a score of 3 points or higher was obtained. According to the presence/absence of frailty, our population was analyzed globally as well as by the degree of dependence using the Barthel scale, and the presence of dementia was assessed by a geriatrician.23
Echocardiographic data were obtained using a Philips Sonos 5500 Ultrasound System (Andover, MA, United States). All patients underwent a comprehensive examination conducted by an experienced echocardiographer (M. Ramos). The severity of aortic valve impairment was assessed following the recommendations of the European Society of Cardiology to evaluate the medium and maximum aortic gradients, aortic valve area, and integral relation.19 Arterial pulmonary hypertension was considered significant when the tricuspid gradient was ≥ 35mmHg.24 We considered left ventricular ejection fraction to be preserved when this fraction was> 50%.
The primary endpoint of our study was all-cause mortality. Follow-up was carried out in the outpatient cardiology department and/or by a telephone interview with the patient or his/her relatives. The duration of the follow-up period for assessing mortality was from the moment of the echocardiographic diagnosis until June 2017. The underlying etiology of mortality was assessed using electronic medical records or death reports. The following clinical assessment data were also collected: a) the onset of symptoms due to AS (syncope, dyspnea, chest pain); b) hospital admission for congestive heart failure; and c) therapeutic changes, such as AVR or TAVI. Patients who developed symptoms were reassessed by the Heart Team.
Congestive heart failure admission was defined as acute inpatient hospitalization with a principal diagnosis of heart failure according to the International Statistical Classification of Diseases and Related Health Problems (ICD-10).
The protocol for this research project was approved by the Alfonso X el Sabio University Ethics Committee and conforms to the provisions of the Declaration of Helsinki. Informed written consent was provided by the study participants.
Statistical analysisThe data were analyzed by the statistical software SPSS version 21.0 (Statistical Package for the Social Sciences, Inc, Chicago, IL, United States). The qualitative variables are presented as absolute and relative frequencies and percentages. For quantitative variables, normality was assessed with the Kolmogorov-Smirnov test, and the mean and standard deviation were then calculated. The qualitative variables were compared using the Pearson chi-square test with Yates’ correction or Fisher's exact test. The quantitative variables were compared using Student's t test for independent samples.
The association between the baseline variables and subsequent mortality is summarized as hazard ratios (HRs) and their 95% confidence intervals (95%CI), obtained by Cox regression. The variables associated with mortality (P<.10) in the univariate analysis were selected for the multivariate analysis, which used a backward stepwise procedure to identify the variables independently associated with mortality. Survival curves were calculated according to frailty and a Charlson comorbidity index ≥ 5. Patients who survived to the end of follow-up were treated as censored.
A cutoff point to predict mortality from the value of the Charlson comorbidity index using the receiver operating characteristic curve was calculated by assessing the area under the curve and sensitivity, specificity, positive predictive, and negative predictive values, all with 95%CI.
RESULTSBaseline characteristicsA total of 104 patients were recruited. The mean age of the participants was 83.3±8.8 years [range, 70-103]; 62 (59.6%) patients met the frailty criteria.
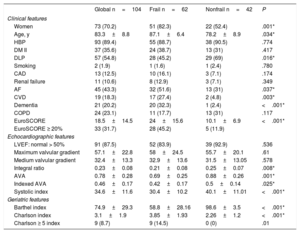
The baseline characteristics according to frailty status are shown in table 1. Patients with frailty were older, and the prevalence of women was higher (82.3%) than in nonfrail patients. Regarding the presence of cardiovascular risk factors, frail patients had a lower prevalence of dyslipidemia than nonfrail patients, with a statistically significant difference (P=.016). A high prevalence of comorbidities was found in the general population; frail patients had higher rates of atrial fibrillation, a history of cerebrovascular disease, and dementia than nonfrail patients. Among the frail patients, 45% had a logistic EuroSCORE higher than 20% (24±15.6; P <.001)
During the follow-up period, 17 patients developed symptoms (dyspnea, angina, or syncope) (16.3%); 47 (45.2%) were hospitalized for congestive heart failure, and 40 (38.5%) remained asymptomatic. At follow-up, among the patients who had symptoms or required CHF hospitalization, 38 (59.4%) remained under medical treatment for AS, 19 (29.7%) underwent TAVI, and 7 (10.9%) underwent AVR surgery. The reasons for continuing conservative treatment after the development of symptoms were Heart Team rejection due to significant frailty, dependence or severe comorbidity (30, 78.9%) or patient refusal (8, 21.1%). Of the 7 patients referred for AVR during follow-up, only 1 died. Four (21%) of the patients referred for TAVI were frail, and none died during follow-up.
Frail patients were associated with a lower aortic valve area, indexed aortic valve area, systolic index, and integral ratio than nonfrail patients (table 1). No statistically significant differences were found in the aortic gradients or the left ventricular ejection fraction.
Clinical, geriatric and echocardiographic characteristics of the global study population
| Global n=104 | Frail n=62 | Nonfrail n=42 | P | |
|---|---|---|---|---|
| Clinical features | ||||
| Women | 73 (70.2) | 51 (82.3) | 22 (52.4) | .001* |
| Age, y | 83.3±8.8 | 87.1±6.4 | 78.2±8.9 | .034* |
| HBP | 93 (89.4) | 55 (88.7) | 38 (90.5) | .774 |
| DM II | 37 (35.6) | 24 (38.7) | 13 (31) | .417 |
| DLP | 57 (54.8) | 28 (45.2) | 29 (69) | .016* |
| Smoking | 2 (1.9) | 1 (1.6) | 1 (2.4) | .780 |
| CAD | 13 (12.5) | 10 (16.1) | 3 (7.1) | .174 |
| Renal failure | 11 (10.6) | 8 (12.9) | 3 (7.1) | .349 |
| AF | 45 (43.3) | 32 (51.6) | 13 (31) | .037* |
| CVD | 19 (18.3) | 17 (27.4) | 2 (4.8) | .003* |
| Dementia | 21 (20.2) | 20 (32.3) | 1 (2.4) | <.001* |
| COPD | 24 (23.1) | 11 (17.7) | 13 (31) | .117 |
| EuroSCORE | 18.5±14.5 | 24±15.6 | 10.1±6.9 | <.001* |
| EuroSCORE ≥ 20% | 33 (31.7) | 28 (45.2) | 5 (11.9) | |
| Echocardiographic features | ||||
| LVEF: normal > 50% | 91 (87.5) | 52 (83.9) | 39 (92.9) | .536 |
| Maximum valvular gradient | 57.1±22.8 | 58±24.5 | 55.7±20.1 | .61 |
| Medium valvular gradient | 32.4±13.3 | 32.9±13.6 | 31.5±13.05 | .578 |
| Integral ratio | 0.23±0.08 | 0.21±0.08 | 0.25±0.07 | .008* |
| AVA | 0.78±0.28 | 0.69±0.25 | 0.88±0.26 | .001* |
| Indexed AVA | 0.46±0.17 | 0.42±0.17 | 0.5±0.14 | .025* |
| Systolic index | 34.6±11.6 | 30.4±10.2 | 40.1±11.01 | <.001* |
| Geriatric features | ||||
| Barthel index | 74.9±29.3 | 58.8±28.16 | 98.6±3.5 | <.001* |
| Charlson index | 3.1±1.9 | 3.85±1.93 | 2.26±1.2 | <.001* |
| Charlson ≥ 5 index | 9 (8.7) | 9 (14.5) | 0 (0) | .01 |
AF, atrial fibrillation; AVA, aortic valvular area, CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; DLP, dyslipidemia; DM II, diabetes mellitus; HBP, high blood pressure; LVEF, left ventricular ejection fraction.
Data are expressed as No. (%) or mean±standard deviation.
The geriatric characteristics are shown in table 1. Frail patients were more dependent according to the Barthel scale (P <.001) and had a higher degree of comorbidities than nonfrail patients. Among the frail patients, 14.5% had a Charlson comorbidity index ≥ 5 (P <.001).
Interestingly, 49 (38.5%) patients who remained asymptomatic at the end of the study had an estimated Barthel scale value of 82.9±24.39 and a Charlson comorbidity index of 1.8±1.6, and 50% met the frailty criteria.
MortalityDuring the median follow-up time of 2.86 [0.5-6.6] years, 47 patients [45.2%] died, with a median time to death of 4.4 [3.75-5.18] years. The overall survival rate was 84% at 1 year, 70% at 2 years, 62% at 3 years, 57% at 4 years and 42% at 5 years. The most frequent causes of death were heart failure/shock (23, 48.9%), respiratory infection (11, 23.4%) and other noncardiac causes (13, 27.7%). No cases of sudden death, ventricular tachycardia or fibrillation were documented in this cohort.
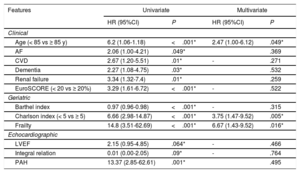
Age and most of the biomedical, geriatric and echocardiographic variables were associated with mortality in the univariate analyses. In the multivariate analysis, the independent clinical variables associated with time to death were age, Charlson comorbidity index ≥ 5, and frailty, which was the most important factor (HR, 6.67; 95%CI, 1.43-9.52) (table 2).
Independent predictors of mortality (multivariate analysis with Cox regression)
| Features | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Clinical | ||||
| Age (< 85 vs ≥ 85 y) | 6.2 (1.06-1.18) | <.001* | 2.47 (1.00-6.12) | .049* |
| AF | 2.06 (1.00-4.21) | .049* | .369 | |
| CVD | 2.67 (1.20-5.51) | .01* | - | .271 |
| Dementia | 2.27 (1.08-4.75) | .03* | .532 | |
| Renal failure | 3.34 (1.32-7.4) | .01* | .259 | |
| EuroSCORE (< 20 vs ≥ 20%) | 3.29 (1.61-6.72) | <.001* | - | .522 |
| Geriatric | ||||
| Barthel index | 0.97 (0.96-0.98) | <.001* | - | .315 |
| Charlson index (< 5 vs ≥ 5) | 6.66 (2.98-14.87) | <.001* | 3.75 (1.47-9.52) | .005* |
| Frailty | 14.8 (3.51-62.69) | <.001* | 6.67 (1.43-9.52) | .016* |
| Echocardiographic | ||||
| LVEF | 2.15 (0.95-4.85) | .064* | - | .466 |
| Integral relation | 0.01 (0.00-2.05) | .09* | - | .764 |
| PAH | 13.37 (2.85-62.61) | .001* | .495 | |
95%CI, confidence interval; AF, atrial fibrillation; CVD, cerebrovascular disease; HR, hazard ratio; LVEF, left ventricular ejection fraction; PAH, pulmonary arterial hypertension.
The survival analysis showed that 69.4% of frail patients died, while only 9.5% of nonfrail patients died. The median time to death for the frail patients was 2.52 years (95%CI, 1.36-3.69). The time to death for nonfrail subjects was not obtained (figure 1). The overall survival rates in these patients were 76% at 1 year, 54% at 2 years, 62% at 3 years, 32% at 4 years, and 15% at 5 years.
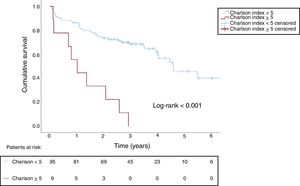
Likewise, a survival analysis was performed using a Charlson comorbidity index value ≥ 5 as the cutoff point; 8.7% of patients met this value, and of those, 100% died during follow-up. The median survival time was 1.01 years (95%CI, 0.36-1.67). Moreover, 66.6% of mortality was due to a noncardiac cause in these patients (figure 2).
Cumulative survival according to the Charlson index: Patients with Charlson index <5 (continuous blue line) and Charlson index ≥ 5 (discontinuous red line) in patients with asymptomatic severe aortic stenosis. The median time elapsed from the diagnosis of severe aortic stenosis to death was 1.01 years in patients with Charlson index ≥ 5.
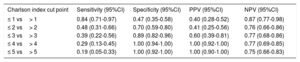
The area under the receiver operating characteristic curve of the Charlson comorbidity index was 0.739 (95%CI, 0.646-0.832) (figure 3). The sensitivity, specificity, positive predictive values and negative predictive values of the Charlson comorbidity index related to mortality are shown in table 3.
Sensitivity and specificity of the Charlson index as a discriminator of mortality
| Charlson index cut point | Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) |
|---|---|---|---|---|
| ≤ 1 vs> 1 | 0.84 (0.71-0.97) | 0.47 (0.35-0.58) | 0.40 (0.28-0.52) | 0.87 (0.77-0.98) |
| ≤ 2 vs> 2 | 0.48 (0.31-0.66) | 0.70 (0.59-0.80) | 0.41 (0.25-0.56) | 0.76 (0.66-0.86) |
| ≤ 3 vs> 3 | 0.39 (0.22-0.56) | 0.89 (0.82-0.96) | 0.60 (0.39-0.81) | 0.77 (0.68-0.86) |
| ≤ 4 vs> 4 | 0.29 (0.13-0.45) | 1.00 (0.94-1.00) | 1.00 (0.92-1.00) | 0.77 (0.69-0.85) |
| ≤ 5 vs> 5 | 0.19 (0.05-0.33) | 1.00 (0.92-1.00) | 1.00 (0.90-1.00) | 0.75 (0.66-0.83) |
95%CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
We define, for the first time, several predictors of mortality in asymptomatic geriatric patients with severe degenerative AS, highlighting age, frailty and a Charlson comorbidity index ≥ 5 as predictive factors.
Geriatric populations with severe AS are a major burden due to their substantial health care costs. In the aged population, severe AS may affect up to 4.6% and 8.1% of patients older than 75 and 85 years, respectively.25,26 Our study population showed a high prevalence of frailty (59.6%), moderate to severe degrees of dependence (49%), and associated comorbidities. All of these factors challenge the detection of early symptoms in the elderly population, who frequently experience severe onset of symptoms.27 According to a review by Généreux et al.,28 among populations with asymptomatic severe AS, 1-year and 5-year survival rates range from 67% to 97% and 38% to 83%, respectively. In our study, the overall survival rate was 84% at 1 year and 42% at 5 years. Our results showed a considerably lower survival rate than that reported by Taniguchi et al. (58% at 5 years) in a retrospective analysis.29 This difference can be explained by our increased prevalence of comorbidities as well as the increased age of our population (77.8±9.4 vs 83.3±8.8 years).
Comorbidities are common in elderly patients; they influence risk-benefit analyses of operative risk, late outcomes after interventions, and life expectancy, regardless of valvular disease.30,31 Similar to our results, Martínez-Sellés et al.18 demonstrated that patients with severe AS and a high comorbidity index (Charlson comorbidity index ≥ 5) had a poor short-term prognosis, with a mean survival less than 1.5 years; mortality was mainly related to noncardiac causes and the patients did not seem to benefit from interventional treatment. The mean Charlson comorbidity index in both studies was very similar; however, the number of patients with elevated comorbidities (≥ 5) was higher in the study by Martínez-Sellés et al. than in our study. In addition, it is important to mention that the Martínez-Sellés et al. population was symptomatic and that the age of inclusion was higher (80 vs 70 years) than that in our study, which could also influence the progression of these patients. This index shows an independent association with mortality and mean survival of 1 year in patients with a score ≥ 5. However, it is logical to consider that an elderly patient with 5 or more comorbidities will have a very poor prognosis. Moreover, as we observed in our population, the frail patient group had more comorbidities and greater limitations in daily living activities than the nonfrail patient group, demonstrating the interrelation of these parameters. As Fried32 described, frailty and comorbidities often overlap in elderly subjects, leading to impairment in functional status, resulting in a poor prognosis.
When the Charlson comorbidity index was assessed as a mortality discriminator for these patients, we found an area under the curve <0.75. A value ≥ 4 had a specificity of 100% but low sensitivity. This finding could be related to the limitations of this index in geriatric patients.33 The weight attributed to each of the pathologies does not correlate with the burden of disease that each causes in the geriatric population. Moreover, some prevalent and disabling diseases that cause high mortality in the elderly, such as parkinsonism, depression or ischemic heart disease without infarction, are not included in this index.
The prevalence of frailty was very high in our population; in total, 59.6% met the frailty criteria, compared with 49.3% in a study by Rodríguez et al.34 Measures of frailty, even after adjustment for age and comorbidities, are highly predictive of poor outcomes, including death, incident disability, and hospitalization in patients with cardiovascular disease.10,35 Regarding AS, it has been shown that frailty is associated with increased hospitalization due to heart failure.34 According to our results, the main factors associated with frailty are advanced age, female sex, a high burden of disease, disability, and cognitive impairment.31,36 Frail patients showed reduced low-density lipoprotein cholesterol fractions as a consequence of protein-calorie malnutrition and loss of muscle mass resulting in bed rest, sarcopenia, and prolonged hospital admission. An increased prevalence of atrial fibrillation was observed in our population, resulting in adverse impacts on morbidity and mortality.35
Our results demonstrated that frailty was an independent factor of mortality. In total, 69.4% of frail patients died, with a median survival of 2.52 years (95%CI, 1.36-3.69); the 5-year survival rate was 15%. These results are considerably lower than those of other studies carried out in the global population where frailty was not considered.28,29 The importance of frailty as a prognostic marker has been widely demonstrated, emerging as a vital cardiovascular care tool and a major component in the decision-making process. Currently, a frailty assessment is essential for patients undergoing surgery, especially those who are candidates for TAVI.12,30 Almost 40% to 50% of patients who undergo TAVI show poor health outcomes, either due to death or because their clinical status does not improve.37,38 However, frailty should not be a reason for exclusion in all cases because AS may be a frailty source and there are aspects of frailty that may be reversible. Thus, in our study population, 4 (6.5%) patients with frailty finally underwent TAVI successfully, with survival at the last follow-up. In this regard, the setting for rehabilitative procedures could prevent some episodes of CHF and improve the prognoses of invasive procedures.39
It would be interesting to consider whether the parameters currently used to assess frailty are valid in sick and debilitated patients. Perhaps other markers of advanced frailty or disability (inability to walk, low albumin, dependence in activities of daily living) should be considered for improved risk discrimination.40 Recently, the FRAILTY-AVR study investigators demonstrated that for both TAVI and AVR, the essential frailty toolset, which includes lower extremity weakness, cognitive impairment, anemia and hypoalbuminemia, had the highest predictive value for death at 1 year and was the strongest predictor of worsening disability at 1 year as well as death at 30 days.41
Consistent with other studies, elderly patients were frequently undertreated.42,43 In our population, the main reason was Heart Team rejection due to significant frailty, dependence or severe comorbidities. Only 40.6% of the patients who developed symptoms were referred for invasive treatment. Invasive therapy was carried out in 45% of the PEGASO registry cohort; this proportion is similar to our result.43 In a study by Iung et al.,42 surgery was carried out in 67% of elderly patients with severe symptomatic AS; this percentage higher than ours, but the patients were younger than out patients, and frailty measures were not included in the study. In fact, population survival was affected when patients who developed symptoms were denied invasive treatment.
In our population, patients were not considered for valve replacement due to the absence of symptoms. Notably, when they developed symptoms, they were rejected due to their frail status. The need for a frailty assessment at the time of severe AS diagnosis, regardless of the symptoms, is critical for this population. To date, frailty scoring has been carried out to decide whether to treat symptomatic patients; our results demonstrate that an early evaluation of asymptomatic AS patients could improve mortality and invasive treatment results. In this regard, the use of the FRAIL questionnaire is potentially useful for screening this population.14
We propose a more restrictive attitude regarding elderly patients requiring valvular replacement for improved mortality results. Frailty screening in nonsymptomatic severe AS patients permits the personalization of our approach, whether that involves more aggressive therapy or the application rehabilitation steps to reverse improvable frailty aspects.
Frailty may be difficult to identify.44 Weakness, decreased mobility, and limitation in performing routine physical activities are common in frail patients but could also be a consequence of AS. Therefore, the presence of frailty in patients who report being asymptomatic due to possible adaptation to their condition could be a manifestation of AS. Considering this evidence, it is crucial to develop multidisciplinary teams focused on geriatric cardiac patients. Future studies are needed based on specific programs that help to reverse frailty and improve the results of invasive treatment in this population.
LimitationsThis study has some limitations. The sample size was modest but compares well to other natural history studies of valvular heart disease. The patients were recruited from a hospital with a high geriatric population, which could be associated with increased dependence and frailty in the study population. The FRAIL questionnaire was used to evaluate frailty; however, this instrument is not the most precise index and may have overestimated the number of frail patients. In our case, this questionnaire was the most appropriate tool because of its simplicity and reliability as a screening test. Moreover, based on other studies,35 we classified the patients as frail and nonfrail, facilitating the analysis. We did not include prefrail patients who were in a state prior to frailty and for whom the intervention could also modify prognosis. The integration of a multidisciplinary team for better geriatric assessment of patients would have been desirable to avoid interviewer subjectivity and bias. Furthermore, during follow-up, some patients eventually underwent AVR surgery or TAVI, which influenced the course of the disease and the outcomes or mortality. Similarly, the patients who developed symptoms were denied invasive treatment, potentially affecting the survival of the population.
The logistic EuroSCORE has been proven to be insufficient to predict events in elderly patients with severe AS. In this regard, the EuroSCORE II seems to be more reliable; however, it was not yet available when we started recruitment.45
Many of the patients were not able to undergo stress tests due to a lack of adaptation to the treadmill, joint disorders or visual impairment, so only a small number of patients underwent this test. Equally, natriuretic peptides were not routinely measured in the patients. Although they may be helpful in decision-making, they are not very specific in the elderly population and should be used cautiously.46
Patients who had not died at the end of the study were treated as censored. Finally, we were surprised that there were no recorded episodes of arrhythmias or sudden death. Perhaps elderly patients have a different disease progression. Comorbidities and frailty could play a role in modifying classical cardiac causes of death associated with degenerative aortic disease.
CONCLUSIONSAmong the risk factors that influence the elderly population with asymptomatic severe AS, frailty is a common condition and was one of the main reasons for denying invasive treatment when necessary. This geriatric syndrome was a powerful independent factor of mortality and poor prognosis. Mortality was also associated with age and a Charlson comorbidity index ≥ 5, but this scale is not sensitive in geriatric patients.
FUNDINGThis work was partially supported by grants (to M. Ramos and M. Quezada) from the VII Convocatoria del Banco de Santander and Alfonso X el Sabio University. Additionally, this work was cofinanced by the Ministry of Health of Junta de Andalucía within the framework of the Integrated Territorial Initiative, file number PI-0048-2017, and the European Regional Development Fund (ERDF) within the Operational Program of Andalusia, ERDF 2014-2020.
CONFLICTS OF INTERESTNone declared.
- -
The role of frailty as a tool to guide the decision-making process in symptomatic patients with AS has been widely demonstrated.
- -
The assessment of frailty will allow us to avoid futile procedures and select the best candidates for TAVI.
- -
However, AS may be the cause of frailty, and there are aspects of frailty that may be reversible with appropriate treatment.
- -
Fraily assessment is not normally carried out until the possibility of intervention is considered.
- -
Elderly patients with a large number of comorbidities have a poor short-term prognosis and do not seem to benefit from interventional treatment.
- -
There is a high prevalence of frailty in elderly patients with severe AS, despite a lack of limiting symptoms. Frailty may mask symptoms in this population and could be a manifestation of advanced disease.
- -
Frailty is a marker of mortality and poor prognosis irrespective of symptoms.
- -
Performing a frailty screening test at the time of the AS diagnosis to identify candidates for further comprehensive geriatric assessment would be useful.
- -
Identifying frail patients as soon as possible will prompt us to take appropriate measures to reverse frailty.
- -
The Charlson comorbidity index is not very sensitive in this population.