Rhythmia is a new nonfluoroscopic navigation system that is able to create high-density electroanatomic maps. The aim of this study was to describe the acute outcomes of atrial fibrillation (AF) ablation guided by this system, to analyze the volume provided by its electroanatomic map, and to describe its ability to locate pulmonary vein (PV) reconnection gaps in redo procedures.
MethodsThis observational study included 62 patients who underwent AF ablation with Rhythmia compared with a retrospective cohort who underwent AF ablation with a conventional nonfluoroscopic navigation system (Ensite Velocity).
ResultsThe number of surface electrograms per map was significantly higher in Rhythmia procedures (12 125 ± 2826 vs 133 ± 21 with Velocity; P < .001), with no significant differences in the total procedure time. The Orion catheter was placed for mapping in 99.5% of PV (95.61% in the control group with a conventional circular mapping catheter; P = .04). There were no significant differences in the percentage of PV isolation between the 2 groups. In redo procedures, an ablation gap could be identified on the activation map in 67% of the reconnected PV (40% in the control group; P = .042). The measured left atrial volume was lower than that calculated by computed tomography (109.3 v 15.2 and 129.9 ± 13.2 mL, respectively; P < .001). There were no significant differences in the number of complications.
ConclusionsThe Rhythmia system is effective for AF ablation procedures, with procedure times and safety profiles similar to conventional nonfluoroscopic navigation systems. In redo procedures, it appears to be more effective in identifying reconnected PV conduction gaps.
Keywords
Catheter ablation is increasingly used to control heart rhythm in patients with drug-refractory atrial fibrillation (AF).1 This procedure is generally guided by nonfluoroscopic 3-dimensional navigation to increase the precision of catheter manipulation, characterize the arrhythmic substrate, and facilitate arrhythmia mapping.
The Rhythmia system (Boston Scientific, Marlborough, Massachusetts, United States) is a new navigator that is able to rapidly create high-density electroanatomic maps by combining a multipolar diagnositic catheter (IntellaMap Orion, Boston Scientific, Marlborough, Massachusetts, United States) with software that automatically acquires the location and signals from recorded local surface electrograms. The distal end of the Orion catheter houses 64 electrodes distributed on 8 small flexible splines (8 electrodes each) arranged as a min-basket with a diameter ranging from 3mm (fully closed) to 22mm (fully deployed). The Rhythmia system combines 2 location technologies: magnetic tracking enables detection of magnetic sensors within the Orion catheter and the Intella Nav OI ablation catheter (Boston Scientific, Marlborough, Massachusetts, United States), whereas impedance tracking can detect any other type of catheter once an initial impedance map has been completed with the Orion catheter. The system automatically calculates map volume.
Little information is contained in the medical literature on the ability of this new system to create precise electroanatomic maps and activation maps; however, preliminary data from animal studies and small patient series indicate that Rhythmia can be used to guide AF ablation procedures and to confirm electrical isolation of the pulmonary veins (PV) after radiofrequency ablation.2–4
The aim of this study was to describe the Rhythmia procedure and acute outcomes observed during initial experience with Rhythmia-guided AF ablation. These results were compared with those obtained in a retrospective control cohort who underwent AF ablation with a conventional nonfluoroscopic navigation system. We also compared left atrial volumes derived from Rhythmia electroanatomic maps with volumes determined by contrast cardiac computed tomography (CT).
METHODSStudy PopulationAn observational study was conducted with the first 62 consecutive patients to undergo Rhythmia-guided ablation of paroxysmal or persistent symptomatic AF (n = 37 and n = 25, respectively) at our center between July 2015 and July 2016. The results were compared with those obtained in a retrospective cohort of consecutive patients who underwent AF ablation as the sole intervention with a conventional navigation system (Ensite Velocity, St. Jude Medical, St. Paul, Minnesota, United States); procedures in the control group were conducted between July 2015 and July 2016 by the same senior system operator who performed the Rhythmia procedures. Mean participant age was 63 years, 46% of the patients were women, and 17 patients had undergone a previous AF ablation procedure, 2 with Rhythmia and 15 with another navigation system. All patients were under anticoagulant therapy for at least 21 days before the procedure. Vitamin K antagonist therapy was maintained without interruption, whereas treatment with new anticoagulants was suspended for 12 to 24hours before ablation and reinitiated on the same day as treatment with no bridging therapy.
The study was approved by the local ethics committee and conformed to the ethical principles of the Helsinki Declaration. All study participants gave informed consent before the procedure.
Rhythmia-guided Ablation ProcedurePreprocedure EvaluationThe day before the ablation procedure, all patients underwent a CT examination, and left atrial anatomy was characterized by segmentation and 3-dimensional reconstruction with the ITK-SNAP220 software application.5 The CT data were used to calculate the atrial volume, excluding the PV and the left atrial appendage. All procedures were conducted under conscious sedation. Patients with active AF underwent electrical cardioversion before the start of the procedure.
Mapping StrategyFor pacing, a conventional diagnostic decapolar catheter was positioned in the coronary sinus, providing an electrical and anatomical reference for impedance tracking. The Orion catheter and the ablation catheter were inserted into the left atrium by double transseptal puncture through deflectale sheaths (Agilis, St. Jude Medical, Minnesota, United States). After transseptal puncture, unfractionated heparin was administrated according to an established protocol6 to maintain an activated clotting time of 300 to 350 s for the duration of the procedure, and heparinized serum was infused (1 U/mL) via the central Orion catheter channel at 1 mL/min to prevent thrombus formation, in accordance with the manufacturer's recommendations.
The left atrium was mapped with the Orion catheter during atrial pacing from the proximal coronary sinus at a cycle length of 550 ms. The system automatically selected appropriate heartbeats and electrograms according to predefined criteria. The initial criteria were as follows: stability over the cycle length with ± 10 ms tolerance; propagation reference with ±5 ms tolerance; heartbeat capture only during the expiratory phase of the respiratory cycle; Motion = 1 mm; Stability = 0.25; Tracking = 3.
For the voltage maps, low voltage areas were defined as those with electrograms < 0.3 mV and normal voltage areas as those with with electrograms > 0.5 mV.
Although a reference CT scan was performed in all patients, during the ablation procedure the system operator was blinded to CT-determined atrial volume. The Rhythmia system automatically calculated atrial volume from the generated map, excluding the PV and left atrial appendage.
With all patients, particular care was taken to maneuver and rotate the Orion catheter slowly at the level of the PV antrum and the left ridge in order to produce a very high point-density map of this region. The septal region was explored by smoothly withdrawing the maximally deployed Orion catheter until meeting resistance from the interatrial septum.
Ablation StrategyFor ablation we used either a Blazer OI catheter or an Intella Nav OI catheter (both from Boston Scientific, Marlborough, Massachusetts, United States). Patients with no previous AF ablation underwent circumferential PV isolation by standard point-by-point ablation, creating wide ablation circles around the left-side and right-side PV and additional lines between the veins. Patients with a previous PV isolation were evaluated by activation mapping for PV entrance and exit and by voltage mapping; conduction gaps in reconnected PV were identified as the earliest PV areas activated at a voltage > 0.3 mV, and focal ablation was conducted at this level.
During PV isolation, the Orion catheter was positioned in the proximal region of the target vein and expanded to its recording position. If this positioning resulted in mechanical or electrical interference with the ablation catheter, the Orion catheter was moved distally.
In all patients, isolation was confirmed by introducing the Orion catheter into the PV and then expanding it to its maximum diameter or until observing deformation of the catheter mini-basket due to contact with the vein walls. When entrance block was confirmed, stimulation from the equatorial electrodes of the Orion catheter was used to confirm exit block. When necessary, left atrium pacing maneuvers were performed from the left atrial appendage and from the right atrium to unmask far-field potentials. In all patients, adenosine boluses were introduced through the Orion catheter into each isolated vein, and focal ablation was performed if reconnection was observed. In all patients, isolation was confirmed a minimum of 20minutes after the last application.
After withdrawal, the Orion catheter tip was systematically checked for thrombi, and all patients had a postprocedure transthoracic echocardiography examination.
Ensite Velocity-guided Ablation ProcedureIn the control group, we followed our usual ablation protocol based on published methodology.7,8 In brief, patients underwent double transseptal puncture followed by electroanatomic mapping guided by the Ensite Velocity system (St. Jude Medical, St. Paul, Minnesota, United States); the other catheters used were a TactiCath irrigated ablation catheter and a Reflexion Spiral variable radius circular diagnostic catheter. The Ensite Verismo software package was used to fuse electrical maps with 3-dimensional reconstructions derived from segmented left atrium contrast CT images. Protocols and techniques for sedation, anticoagulant therapy (intraprocedural and periprocedural), and ablation were the same as those used in the Rhythmia group.
Statistical AnalysisQuantitative variables are presented as means ± standard deviation. Categorical variables are presented as number and percentage. Differences between 2 patient groups were analyzed by the Student t test for independent samples. Qualitative variables were compared by the chi-square test or the Fisher exact test. In bilateral tests, statistical significance was assigned at P < .05. Statistical analysis was conducted with SPSS for Windows, version 22 (SPSS Statistics, IBM Software Group).
RESULTSA total of 124 patients underwent PV isolation: 62 (50%) guided by the Rhythmia system and 62 (50%) by the Ensuite Velocity system, used as a control. There were no significant between-group differences in preprocedure characteristics (Table).
Preprocedure Patient Characteristics
| Patient characteristics | Rhythmia (n = 62) | Velocity (n = 62) | P |
|---|---|---|---|
| Age, y | 63.84 | 64.61 | .694 |
| Sex, M/F | 34/28 | 14/21 | .199 |
| Type of atrial fibrillation, paroxysmal/persistant | 37/25 | 39/23 | .712 |
| Redo procedure, no. (%) | 17 (27.41) | 18 (29.03) | .842 |
| Median CHA2DS2-VASc score | 2 | 2 | .783 |
| Left ventricular ejection fraction, % | 64.73 | 62.81 | .104 |
| Left atrium diameter, mm | 40.28 | 41.51 | .322 |
| Standard pulmonary vein anatomy, no. (%) | 47 (75.80) | 49 (79.03) | .668 |
CHA2DS2-VASc: congestive heart failure, hypertension, age ≥ 75 years (doubled), diabetes mellitus, prior strok (doubled), vascular disease, age 65 to 74 years, and sex (female)
F, female; M, male.
In the Rhythmia group, 45 patients underwent index ablation and 17 had redo procedures. Preprocedure CT identified 4 independent PV in 47 patients (75.8%) and variant anatomies in 15 patients (24.1%): 6 with a left common PV, 1 with a right common PV, 1 with an inferior common PV, and 7 with a right middle PV. This pattern did not differ significantly from the control group, among whom 49 patients (79%) had 4 independent PV and 13 (20.9%) had variant anatomies (P = .668).
The Orion catheter could be properly positioned in the interior of 246 of the 247 PV in the Rhythmia group (99.59%). Mapping with the Orion catheter proved impossible in only 1 small right inferior PV with a CT-determined diameter of 5 mm; mapping of this vein was possible with the ablation catheter. In the control group, the circular catheter was properly positioned inside 240 of the 251 PV present (95.61%), which is a significantly lower success rate than achieved with the Orion catheter (P = .04).
The mean number of valid points per map was 12 125 ± 2826 in the Rhythmia group and 133 ± 21 in the control group (P < .001). The mean time for left atrium mapping with the Orion catheter was 18 ± 5 minutes. Electrical isolation was achieved in 245 of 246 PV mapped in the Rhythmia group (99.5%) and in all 240 mapped PV in the control group, with no statistically significant differences (P = .8).
In the Rhythmia subgroup undergoing repeat ablation, 68 PV were examined, 28 (41%) of them showing reconnection. In 19 of the 28 reconnected PV (67%), the activation map revealed a clear conduction gap, and a single focal ablation achieved vein isolation or evident delay in the electrograms and an altered entrance pattern. In the remaining reconnected veins (9 of 28), the activation map did not identify a successful ablation point: either there was no clear conduction gap (6 of 28) or focal ablation in the presumptive gap did not achieve vein isolation (3 of 28). In the control group, initial electrical activation with the circular catheter identified clear conduction gaps in only 10 of the 25 reconnected PV (40%) according to the criteria defined above (P = .042).
There were no significant between-group differences in mean procedure time (149 ± 21 minutes in the Rhythmia group vs 145 ± 30 minutes in the control group; P = .412). Total fluoroscopy time was significantly higher in the Rhythmia group than in the control group (28 ± 9minutes vs 24 ± 7 minutes; P = .038). However, comparison of the last 31 patients examined in each group revealed no significant differences (25 ± 9 minutes in the Rhythmia group vs 24 ± 9 minutes in the control group; P = .722), indicating that the overall difference likely reflects a learning curve effect.
Mean atrial volume measured with the Rhythmia system was significantly smaller than the volume estimated by CT (109.3 ± 15.2mL vs 129.9 ± 13.2 mL; P < .001).
Postprocedure echocardiography in 1 patient in the Rhythmia group detected pericardial bleeding without cardiac taponade, which became sufficiently severe to require treatment by pericardiocentesis. Another patient developed significant groin hematoma, which was resolved by sustained local compression. In the control group, cardiac taponade was observed in 1 patient, who was treated by pericardiocentesis, and another patient had pericarditis without bleeding. There were no thromboembolic complications or between-group differences in the total number of complications.
DISCUSSIONTechnical developments introduced to simplify AF ablation procedures include 3-dimensional mapping systems and their integration with other cardiac imaging techniques. By automatically annotating recorded electrograms, high-density mapping systems can generate electroanatomic maps with a high point-density in a short time. The series presented here is the largest published to date on procedures guided by the Rhythmia system, and this study is the first to compare Rhythmia with a conventional nonfluoroscopic navigation system for AF ablation procedures. This is also the first study to validate the anatomical precision of the Rhythmia system in a clinical context, through the quantification of heart chamber volume calculations.
System StrengthsOur series confirms the ability of the Rhythmia system to create a very high point-density map of the left atrium and PV without interfering in the AF ablation procedure workflow. This allowed us to achieve reasonable procedure times even in an initial study that included learning how to operate the system. In all patients, maps obtained with Rhythmia were comparable with those obtained by CT. When used together with the multipolar Orion catheter, the Rhythmia system allows rapid generation of complete activation and voltage maps without the need for manual correction. Using Rhythmia in automatic mode, we obtained maps with 12 125 electrograms on average in a mean mapping time of 18minutes. In most instances, manual correction is unnecessary because the system compares each point with its neighbors and corrects discordances due to poorly recorded isolated points. This feature ensures that a small number of erroneously annotated points will not affect overall map interpretation. In our series, the high resolution of the Rhythmia activation maps was especially useful in redo proceures because it enabled more accurate identification of reconnection gaps than the conventional navigation system (gaps detected in 67% of reconnected PV with Rhythmia vs 40% withVelocity; P = .042) (Figure 1). Similarly, after apparently complete circumferential ablation around a vein in a patient undergoing an index ablation, the Rhythmia system could generate a new activation map of a single vein within 2minutes, providing a guide for focal ablation. For several patients in our series who underwent redo ablation, we created “exit maps” of reconnected PV by in-vein stimulation with the ablation catheter and deployment of the Orion catheter in the venous ostium, followed by withdrawal toward the atrium (Figure 2 and video of the supplementary material). This maneuver takes only a few minutes and is very useful for delimiting reconnection gaps; however, its value is limited when stable capture within the PV cannot be achieved.
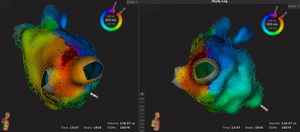
Example images from an atrial fibrillation patient undergoing redo ablation, in whom atypical atrial flutter was induced mechanically. An 18 579-point activation map was created in 13 minutes. A block line made in the previous procedure can be seen on the roof of the left atrium, with a conduction gap where it joins the right superior pulmonary vein. The arrhythmia resolved after focal ablation at this level. Beats, beats captured for map creation; EGMs, electrograms captured for map creation; Time, mapping time; Volume, map volume.
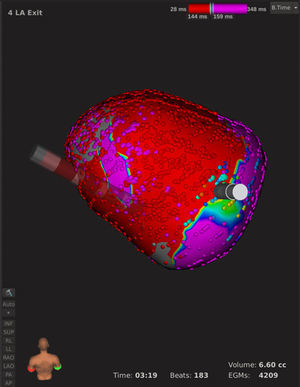
Left superior pulmonary vein exit map from a posterior viewpoint in an atrial fibrillation patient undergoing redo ablation. During pacing from the ablation catheter inside the pulmonary vein, the maximally deployed Orion catheter was withdrawn slowly with rotation. A clear conduction gap can be seen in the PV antrum. Electrical isolation was achieved by focal ablation at this point. Beats, beats captured for map creation; EGMs, electrograms captured for map creation; Time, mapping time; Volume, map volume.
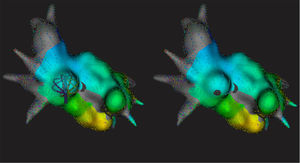
In our view, the combination of the Orion catheter with a deflectale sheath creates a very versatile tool for left atrium and PV mapping, allowing adequate navigation even in patients with a variant anatomy and providing a number of advantages over conventional circular mapping catheters (Figure 3). For example, the number of selectively catheterized PV was significantly higher than that achieved with a conventional circular catheter, and the Orion catheter adapted to a wide range of venous anatomies in acceptable mapping times. Another advantage of the Orion catheter is its aptness for exploring the septal region close to the inferior portion of the right inferior PV, a site that presents difficulties for both mapping and ablation due to the risk of displacement to the right atrium. By smoothly withdrawing the fully-deployed catheter until consistently encountering resistance from the interatrial septum, the resulting map is left with an approximately 3 mm diameter zone lacking recorded potentials, corresponding to the transseptal puncture site (Figure 4). The shape of the Orion catheter also facilitates mapping of the venous ostia by the simple maneuver of deploying it within the vein and withdrawing it slowly until a typical “spike” is observed on passing into the left atrium. Finally, the pairing of electrodes both longitudinally and radially on the Orion catheter splines allows left superior PV potentials to be distinguished, without catheter manipulation, from signals due to far-field potentials from the left atrial appendage; this is possible because impulses propagate from proximal to distal in the PV, whereas far-field signals are simultaneous (Figure 5).
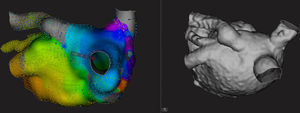
Posterior views of variant pulmonary vein anatomy revealed by electroanatomic mapping (left) and 3-dimensional computed tomography reconstruction (right) of the left atrium. A left superior pulmonary vein opens in the atrial roof, showing evidence of dilatation and a proximal curvature that could interfere with multipolar catheter mapping. The Orion catheter allowed exploration of the 4 veins observed. Mapping time, 24minutes.
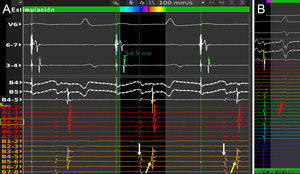
A: Example electrograms recorded with the Orion catheter inside the left superior pulmonary vein; with no need for catheter manipulations or stimulation maneuvers, recordings from a single spline can distinguish pulmonary vein potentials, which propagate from proximal to distal (yellow arrows), from “far-field” potentials originating in the left atrial appendage, which are simultaneous (white arrows). B: Electrograms from far-field ventricular potentials (red arrow) are also simultaneous, allowing them to be distinguished from pulmonary vein potentials.
Many unresolved questions remain. For voltage mapping, there is a need to define the optimal cutoffs for distinguishing between healthy and scar tissue. The values used in the present series satisfactorily revealed lines made in previous procedures; however, we cannot be sure that these values are optimal for characterization of atrial tissue.
Volumes measured with Rhythmia were smaller than those measured by CT, indicating that Rhythmia did not explore the whole left atrium. It should be borne in mind that these Rhythmia maps were made in the clinical setting of AF ablation procedures; areas not explored systematically in this series might be covered by a more detailed mapping procedure of the kind used in patients with left atrial flutter, thus producing values closer to the true atrial volume. Even with this caveat, the recorded volumes were 84.14% of values measured by CT, indicating adequate adaptation by the Orion catheter to the different left atrium regions.
Inclusion of a magnetic sensor significanly improved the introduction of the ablation catheter, but there is room for further improvement in vision-guided catheter placement. It would also be useful to have a magnetic catheter with a resistance sensor. Finally, given the high cost of the Rhythmia system, a cost-benefit analysis is warranted to help prioritize selected patient subgroups for this procedure.
Study LimitationsThe obvious limitations of this study are the lack of randomization in the control group and the lack of long-term follow-up. However, rather than evaluate long-term clinical outcomes, this study aimed to describe experience and acute outcomes obtained with Rhythmia-guided AF ablation procedures. Further, randomized studies will be needed with larger samples in order to evaluate long term clinical efficacy.
CONCLUSIONSThe results of this observational study indicate that AF ablation guided by the Rhythmia system is safe, has similar procedure times to conventional nonfluoroscopic navigation systems, and offers potential benefits over these, especially in patients requiring redo ablation. The Orion catheter, in combination with a deflectable sheath, is easy to maneuver in the left atrium, permitting exploration of more PV territory than conventional circular navigation catheters, even in patients with variant and complex anatomies. Left atrium volume measured with the Rhythmia system is slightly smaller than that calculated from CT analysis. Clinical translation of the potential advantages of this system should be explored in studies designed for that purpose.
CONFLICTS OF INTERESTNone declared.
- –
Rhythmia is the first of a new generation of nonfluoroscopic navigation systems that produce high-density maps through a combination of a multipolar diagnostic catheter with software able to automatically acquire information from recorded electrograms.
- –
Data from small series indicate the likely usefulness of this system for guiding AF ablation procedures; however, to date, there have been no studies with a comparison group.
- –
Although Rhythmia can create high-density activation maps, previous reports have not explored its ability to define PV reconnection gaps in AF procedures.
- –
This is the largest study to date on Rhythmia-guided procedures on any substrate and presents the first comparison with conventional navigation systems in AF ablation.
- –
This is the first study to validate the anatomical precision of the Rhythmia system, through the quantification of heart chamber volume calculations.
- –
Rhythmia-guided high-resolution activation maps are useful for locating reconnection gaps in redo procedures.
- –
The study describes the technique for creating exit maps for reconnected PV in redo AF ablation procedures.