Improvements in survival among cancer patients have revealed the clinical impact of cardiotoxicity on both cardiovascular and hematological and oncological outcomes, especially when it leads to the interruption of highly effective antitumor therapies. Atrial fibrillation is a common complication in patients with active cancer and its treatment poses a major challenge. These patients have an increased thromboembolic and hemorrhagic risk but standard stroke prediction scores have not been validated in this population. The aim of this expert consensus-based document is to provide a multidisciplinary and practical approach to the prevention and treatment of atrial fibrillation in patients with active cancer. This is a position paper of the Spanish Cardio-Oncology working group and the Spanish Thrombosis working group, drafted in collaboration with experts from the Spanish Society of Cardiology, the Spanish Society of Medical Oncology, the Spanish Society of Radiation Oncology, and the Spanish Society of Hematology.
Keywords
Advances in early cancer diagnosis and treatment have significantly improved survival in recent decades. At the same time, concerns about the clinical repercussions of cancer therapy on the cardiovascular (CV) system1 have raised awareness of the importance of a multidisciplinary approach in this setting. The purpose of Cardio-Onco-Hematology (C-O-H) teams is to facilitate the treatment of oncology-hematology by establishing prevention and early treatment strategies for the various manifestations of cardiotoxicity.2 This document aims to present a practical, consensus-based, multidisciplinary approach to preventing, monitoring, and treating atrial fibrillation (AF) in patients with active cancer, defined as patients receiving active cancer treatment, patients diagnosed with cancer in the past year, and patients with metastatic disease. The methodology used to produce this consensus statement is described in the first section of the , and the list of experts who participated in the project and endorse the statement are shown in , together with a list of the scientific societies to which they belong.
Epidemiology of AF in patients with cancerImprovements in cancer prognosis and the advent of targeted therapies have dramatically increased the number of cases of cardiac arrhythmias seen in oncology and hematology, although their incidence is underestimated in the literature due to the exclusion of patients with heart disease from key clinical trials.1,3,4 The mechanisms by which certain cancer treatments trigger arrhythmia have not been fully elucidated, but individual risk varies according to the treatment, the clinical circumstances of the patient, and metabolic and inflammatory changes induced by the tumor.5,6 The risk is higher in patients older than 65 years (almost 2 of every 3 patients with cancer) and in those with pre-existing CV disease (30% of all patients with cancer).1,3,7
AF is the most common sustained arrhythmia in the general population8 and is substantially more common in patients with cancer.1,3,6 Prevalence rates vary greatly from one study to the next, depending on whether the focus was on patients with active cancer, patients with a past history of cancer, or patients undergoing oncologic surgery.1,3 In the REGARDS (REasons for Geographic And Racial Differences in Stroke) study,9 which analyzed 15 428 patients, those with cancer (n=2314, 15%) were 20% more likely to have AF than those without cancer, even when they were not receiving cancer treatment and following adjustment for age, CV disease, and other AF risk factors. Neither AF (past or present) nor AF risk is a contraindication for cancer treatment, but their presence calls for multidisciplinary management by a C-O-H team.2 The occurrence of AF during active cancer therapy (2%-16% of cases depending on the series) is associated with a 2-fold increased risk of a thromboembolic event and a 6-fold increased risk of heart failure.1,3,6,10
Cancer drugs associated with an increased risk of AFA list of cancer drugs that can cause AF is presented in Table 1. The classification of these drugs is shown in , together with their main adverse effects () and potential drug-drug interactions (DDIs) reported at the time of publication of this document ().11,12 Updated DDIs can be checked online using the RxList Drug Interactions Checker,13 the Electronic Medicines Compendium website,14 or the Drug Information Center of the Spanish Agency of Medicines and Healthcare Products (AEMPS).15
Cancer drugs that induce atrial fibrillation
| • Vinca alkaloids |
| • Alkylating agents: cisplatin, cyclophosphamide, ifosfamide, melphalan |
| • Gonadotropin-releasing hormone antagonists: degarelix |
| • Anthracyclines: doxorubicin, mitoxantrone |
| • Monoclonal antibodies: alemtuzumab, cetuximab, ipilimumab, obinutuzumab, ofatumumab, rituximab |
| • Antimetabolites: azathioprine, capecitabine, 5-fluorouracil, gemcitabine, methotrexate, pentostatin |
| • Aromatase inhibitors |
| • Bcr-Abl inhibitors: dasatinib |
| • HER2 inhibitors: trastuzumab |
| • Histone deacetylase inhibitors: vorinostat |
| • Proteasome inhibitors: bortezomib |
| • Protein kinase inhibitors: ibrutinib |
| • Androgen synthesis inhibitors: abiraterone |
| • Type II topoisomerase inhibitors: amsacrine, etoposide |
| • Vascular endothelial growth factor inhibitors: bevacizumab |
| • Interferons |
| • Interleukin 2 |
| • Taxanes: docetaxel, paclitaxel |
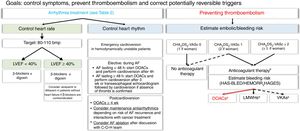
Treatment of AF is similar regardless of whether the patient has active cancer or not. The ultimate goal is to improve symptoms, control arrhythmia, and prevent stroke and systemic embolism.8 It should be noted that standard bleeding and thromboembolic risk stratification scales have not been validated for use in patients with active cancer. In this document, we have maintained the distinction between valvular AF (AF associated with moderate to severe rheumatic mitral stenosis or the presence of mechanic heart valves) and nonvalvular AF as per the European Society of Cardiology Guidelines for the management of AF8 (Figure 1).
Algorithm for treating atrial fibrillation in patients receiving active cancer treatment. The dashed line indicates situations supported by less evidence.
AF, atrial fibrillation; bmp, beats per minute; C-O-H, cardio-onco-hematology; DOACs, direct oral anticoagulants; HAS-BLED, acronym for hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, and drugs or alcohol; HEMORR2HAGES, acronym for hepatic or renal disease, ethanol abuse, malignancy, older [≥ 75 y), reduced platelet count, rebleeding, hypertension, anemia, genetic factors, excessive fall risk, and stroke; LMWHs, low–molecular-weight heparins; LVEF, left ventricle ejection fraction; VKAs, vitamin K antagonists. aCheck possible interactions. bConsider percutaneous left appendage closure for patients with a high bleeding risk and/or contraindications for anticoagulation and a life expectancy of >1 y.
The decision to attempt to control heart rate or heart rhythm should be individually tailored. Whatever the strategy chosen, the aim should be to improve symptoms, minimize the risk of CV complications, prevent interruptions of cancer treatments, and reduce DDIs.1,3,8 The C-O-H team should thus assess the likelihood of maintaining the patient in sinus rhythm based on his or her age, the presence of heart disease, and the active cancer treatment.1,16
Emergency electrical cardioversion should be considered for unstable patients8 (Figure 1). In patients with recent-onset AF, potentially reversible triggers should be corrected to maintain adequate heart rhythm.8
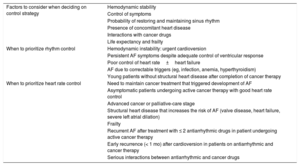
A heart rate control strategy is generally preferred in stable patients, as maintenance of sinus rhythm is unlikely in patients being treated with proarrhythmic cancer drugs.1,3 This strategy is also recommended for elderly patients, patients with CV disease or a severely dilated left atrium, patients with good symptom control, and those with a poor cancer prognosis or in palliative care.1,3 Postoperative AF requires special attention.6,17 A heart rhythm control strategy should be contemplated in patients without CV disease or risk factors once potential triggers (eg, heart failure, anemia, infections) have been controlled. A heart rate control strategy, by contrast, would be the first choice for patients with cumulative CV risk factors. The main factors that should be considered when selecting one or other strategy are summarized in Table 2.
Heart rhythm versus heart rate control
| Factors to consider when deciding on control strategy | Hemodynamic stability |
| Control of symptoms | |
| Probability of restoring and maintaining sinus rhythm | |
| Presence of concomitant heart disease | |
| Interactions with cancer drugs | |
| Life expectancy and frailty | |
| When to prioritize rhythm control | Hemodynamic instability: urgent cardioversion |
| Persistent AF symptoms despite adequate control of ventricular response | |
| Poor control of heart rate±heart failure | |
| AF due to correctable triggers (eg, infection, anemia, hyperthyroidism) | |
| Young patients without structural heart disease after completion of cancer therapy | |
| When to prioritize heart rate control | Need to maintain cancer treatment that triggered development of AF |
| Asymptomatic patients undergoing active cancer therapy with good heart rate control | |
| Advanced cancer or palliative-care stage | |
| Structural heart disease that increases the risk of AF (valve disease, heart failure, severe left atrial dilation) | |
| Frailty | |
| Recurrent AF after treatment with ≤ 2 antiarrhythmic drugs in patient undergoing active cancer therapy | |
| Early recurrence (< 1 mo) after cardioversion in patients on antiarrhythmic and cancer therapy | |
| Serious interactions between antiarrhythmic and cancer drugs |
AF, atrial fibrillation.
Advances in ablation techniques and the introduction of new-generation catheters have helped simplify ablation procedures and extended their use to more complex cases.8 C-O-H teams may consider ablation in highly selected patients when other heart rate or rhythm control strategies have failed or when there is a high likelihood of interactions with cancer drugs, assuming of course that ablation is not contraindicated by the cancer prognosis. Although catheter ablation is an effective alternative for treating AF,18 very few studies have analyzed its use in cancer. The procedure, however, was found to be both safe and effective in a retrospective study of 15 patients with refractory AF after pneumonectomy, with 80% of patients in sinus rhythm at 1-year follow-up.19 Note that effective ablation will not prevent anticoagulation in such cases.
Stroke and systemic embolism preventionevaluation of thromboembolic and bleeding risksAs with the general population, estimation of thromboembolic and bleeding risk is also necessary in patients with cancer to determine the need for anticoagulation and assess associated risks.1,3,8 Active cancer is associated with an increased risk of thromboembolic and bleeding events that depends on the characteristics of the tumor (location, histology, stage) and the adverse effects of the cancer drug20–23 (Table 3). Risk of arterial and venous thromboembolic complications is increased by the release of procoagulant and fibrinolytic agents and proinflammatory cytokines,24 while bleeding risk is increased by the tumor, the need for surgery, and cancer treatment-induced myelotoxic effects.8,25
Cancer drugs associated with increased risk of bleeding or thrombosis
| Bleeding | • Vinca alkaloids, alkylating agents, monoclonal antibodies (aflibercept, bevacizumab, ramucirumab, trastuzumab emtansine), antiestrogens, antimetabolites (pentostatin), anthracyclines, bleomycin, campothecins, carfilzomib, epipodophyllotoxins, ibrutinib, BCR-Abl, BRAF, and VEGF/VEGFR inhibitors, interleukins, L-asparaginase, ruxolitinib, taxanes, temozolomide• Cyclophosphamide, ifosfamide: hemorrhagic cystitis• Megestrol, tamoxifen: uterine bleeding |
| Arterial thrombsosis | • Alkylating agents (carboplatin, cyclophosphamide, cisplatin, estramustine, oxaliplatin), monoclonal antibodies (aflibercept, bevacizumab), anthracyclines, antimetabolites (capecitabine, 5-fluorouracil, gemcitabine, methotrexate), EGFR inhibitors (cetuximab), bleomycin, protein kinase inhibitors (axitinib, lenvatinib, pazopanib, sorafenib, sunitinib), proteosome inhibitors (carfilzomib), VEGF inhibitors (aflibercept, bevacizumab, ramucirumab), irinotecan, taxanes, tasonermin, tretinoin |
| Venous thrombosis | • Alkylating agents (carboplatin, carmustine, cyclophosphamide, cisplatin, estramustine, oxaliplatin, temozolomide), gonadotropin-releasing hormone analogs, antiandrogens, monoclonal antibodies (cetuximab, panitumumab), antiestrogens, antimetabolites (capecitabine, 5-fluorouracil, gemcitabine, methotrexate, pentostatin), anthracyclines, eribulin, immunomodulators (lenalidomide, pomalidomide, thalidomide), aromatase inhibitors, BCR-Abl inhibitors (dasatinib, nilotinib, ponatinib), EGFR inhibitors (erlotinib, gefitinib), mTOR inhibitors, proteosome inhibitors (carfilzomib), cyclin-dependent kinase inhibitors (abemaciclib), inhibitors of intracellular kinases that participate in angiogenesis (axitinib, pazopanib, regorafenib, sunitinib), irinotecan, megestrol, progestogens, raloxifene, tamoxifen, tasonermin, tretinoin, vinflunine, vorinostat• Erythropoiesis-stimulating agents and granulocyte colony-stimulating factors |
EGFR, epidermal growth factor receptor; mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
In the absence of specific risk stratification scales, we recommend using the CHAD2S2-VASc (congestive heart failure, hypertension, age ≥ 75 y [double score], diabetes mellitus, stroke [double score] vascular disease, age 65-74 years, and sex [female]) or HAS-BLED (hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol) scoring systems to stratify and periodically reassess thromboembolic and bleeding risk in patients with nonvalvular AF.1,3,8,26 Men with a CHA2DS2-VASc score of 1 or higher and women with a score of 2 or higher should be treated with anticoagulants, unless absolutely contraindicated (Figure 1).8 Because patients with AF and cancer have a higher thromboembolic risk than patients with AF without cancer,25 risk should be estimated on a case-by-case basis in the absence of absolute indication for anticoagulation. Other scales that could help stratify risk in complicated cases are the ABC stroke risk score27 (age, biomarkers [highly-sensitive troponin T measurements and NT-proBNP], and clinical history [prior stroke or transient ischemic attack]) and the HEMORR2HAGES score28 (hepatic or renal disease, ethanol abuse, malignancy, older age [≥ 75 years], reduced platelet count, rebleeding, hypertension, anemia, genetic factors, excessive fall risk, and stroke).
Anticoagulant therapyAlthough anticoagulant therapy requires rigorous management in the setting of cancer, suboptimal prescribing has been reported for patients with active cancer and AF. According to a clinical practice study, one-fourth of patients with this profile were not prescribed anticoagulants while a third were treated with nontherapeutic doses of low–molecular-weight heparins (LMWHs).29
Although there are 3 classes of anticoagulants, data on their use in patients with active cancer and AF are lacking in all cases. Decisions on the choice of anticoagulant should be taken on a case-by-case basis following consideration of the cancer and its prognosis, the risk of thromboembolic and bleeding complications, the pharmacokinetics of the anticoagulant (), the active cancer treatment, potential DDIs, and the likelihood of changes to kidney or liver function during treatment. No interactions have been reported in patients treated with radiation therapy only. Antiplatelets should generally be avoided in patients with active cancer on anticoagulants.1,3,8
Vitamin K antagonistsManagement of both warfarin and acenocoumarol is complicated in patients with AF and active cancer, as both safety and effectiveness depend on a narrow therapeutic window and the likelihood of achieving optimal time in therapeutic range (> 65%)30,31 is reduced by the cancer treatment.1,3,32,33 Concomitant cancer and vitamin K antagonist (VKA) therapy increases thrombotic and bleeding risk due to DDIs and reduces the likelihood of maintaining optimal time in therapeutic range due to malnutrition, vomiting, hepatic failure, thrombocytopenia, and the need for frequent operations.3,34 Therefore, in patients undergoing active cancer treatment, VKAs should be reserved for those with valvular AF and international normalized ratio (INR) values that can be maintained within the target range.
Low–molecular-weight heparinsDespite the lack of evidence supporting the use of LMWHs as a prophylactic treatment for thromboembolism in patients with cancer and AF and the shortage of studies on potential DDIs, LMWHs are often prescribed to patients during active cancer treatment.29 Their long-term use, however, is limited by high costs, loss of quality of life, and complications associated with long-term intravenous therapy. LWMHs may be considered in the event of serious interactions between cancer drugs and oral anticoagulants or poor tolerance of the latter.3,6,24
Direct oral anticoagulantsDirect oral anticoagulants (DOACs) are the treatment of choice for members of the general population with nonvalvular AF, as they are superior to VKAs in terms of efficacy, tolerance, and safety.8,35,36 Their main benefits over other anticoagulants are predictable pharmacokinetics (enabling proper anticoagulation without the need for systematic monitoring), rapid onset of action and a short half-life, availability in oral formulations, fewer interactions with food and other drugs, and availability of reversion agents8,35 ().
Findings from 3 recent randomized clinical trials comparing DOACs with dalteparin in the treatment of venous thromboembolism in patients with cancer showed that edoxaban (HOKUSAI-VTE37), rivaroxaban (SELECT-D38), and apixaban (ADAM-VTE39) were both safer and more efficacious than dalteparin for the prevention of recurrent venous thromboembolism. Accordingly, they are recommended by a number of recent consensus statements as an alternative treatment for cancer-associated venous thromboembolism in the absence of high bleeding risk and interactions with cancer drugs.40–42
The evidence base for the safety and efficacy of DOACs as prophylactic treatment for cerebrovascular stroke and systemic embolism in patients with AF and active cancer is less strong, as these patients were excluded from pivotal trials in the field.8,35 Post-hoc analysis of data from the ARISTOTLE study showed no differences in the incidence of thromboembolic events or major bleeding in patients with or without cancer and showed that apixaban had a more favorable safety and efficacy profile than warfarin in both populations.43 On analyzing 1153 patients diagnosed with cancer following inclusion in the ENGAGE AF-TIMI 38) trial, Fanola et al.44 concluded that edoxaban performed similarly to warfarin in preventing embolic events and did not increase bleeding risk. Compared with warfarin, edoxaban was associated with a 46% risk reduction for the composite of ischemic stroke, systemic embolism, and myocardial infarction in patients with cancer. In addition, no significant differences were observed for drug concentrations or factor X activity between patients with and without cancer.44 Likewise, several clinical practice studies have shown DOACs to be safer and more effective than warfarin in the prevention of stroke and systemic embolism in cancer45–49 (Table 4).
Studies of patients with cancer and atrial fibrillation treated with DOACs
| Effectivenessa (thromboembolic events) | Safety (major bleedingb) | |
|---|---|---|
| Ording et al.46Prospective study of Danish population-based medical records from 2010-2014: 68 119 patients (11 855 with cancer) | Presence vs absence of cancer with VKAs (% risk)Annual incidence: 6.5% vs 5.8%Presence vs absence of cancer with DOACs (% risk)Annual incidence: 4.9% vs 5.1% | Presence vs absence of cancer with VKAs (% risk)Annual incidence: 5.4% vs 4.3%Presence vs absence of cancer with DOACs (% risk)Annual incidence: 4.4% vs 3.1% |
| Melloni et al.43Posthoc ARISTOTLE analysis: 1236 patients with cancer (76 with active cancer also receiving apixaban) | Apixaban vs warfarin in patients with cancer: annual incidence of stroke/SE, 1.4% vs 1.2% (HR=1.09)Apixaban vs warfarin in patients without cancer: annual incidence of stroke/SE, 1.3% vs 1.6% (HR=0.77) | Apixaban vs warfarin in patients with cancer: annual incidence, 2.4% vs 3.2% (HR=0.76)Apixaban vs warfarin in patients without cancer: annual incidence, 2.1% vs 3.1% (HR=0.69) |
| Laube et al.49Single-center prospective study: 163 patients with cancer and AF treated with rivaroxaban (1-y follow-up) | Annual incidence: 1.4% | Annual incidence: 1.2% |
| Russo et al.45Single-center observational study: 76 patients with cancer and AF treated with DOACs) | No thromboembolic events | Cumulative incidence: 3.9%Annual incidence: 1.4% |
| Fanola et al.44ENGAGE AF-TIMI 38: 1153 patients with active cancer (follow-up, 2.8 y). New incident cancer rate, 85.9%; recurrence rate (previous cancer > 5 y), 14.1% | Stroke/SE with high-dose edoxaban vs VKA in cancer: HR=0.60 (0.31-1.15)Ischemic stroke/SE/AMI with high-dose edoxaban vs VKA in cancer: HR=0.54 (0.31-0.93) | Major bleeding with high-dose edoxaban vs VKAs in cancer: HR=0.98 (0.69-1.4) |
| Shah et al.47Retrospective study: 16 096 patients with cancer (6075 treated with DOACs and 10 021 with VKAs; follow-up, 12 mo) | Ischemic stroke:Rivaroxaban vs VKA: HR=0.74 (0.40-1.39)Dabigatran vs VKA: HR=0.89 (0.56-1.42)Apixaban vs VKA: HR=0.71 (0.19-2.60)Venous thromboembolism:Rivaroxaban vs VKA: HR=0.51 (0.41-0.63)Dabigatran vs VKA: HR=0.28 (0.21-0.38)Apixaban vs VKA: HR=0.14 (0.07-0.32) | Major bleedingRivaroxaban vs VKA: HR=1.09 (0.79-1.39)Dabigatran vs VKA: HR=0.96 (0.72-1.27)Apixaban vs VKA: HR=0.37 (0.17-0.79) |
| Vedovati et al.50Multicenter study of AF and DOACs (289 patients with cancer [12.6% of cohort]) | Ischemic stroke, TIA, or SE in cancer vs no cancer with DOACs: HR=2.60 (1.08-6.20) | Major bleeding in cancer vs no cancer with DOACs: HR=2.0 (1.25-3.30) |
| Kim et al.48Retrospective study of AF and recently diagnosed cancer: 2568 patients (P<.001 for all comparisons [propensity score matching]) | Stroke/SE rate with warfarin vs DOACs: 5.9% vs 1.3% a year | Major bleeding with warfarin vs DOACs: 5.1% vs 1.2% a yearDigestive bleeding with warfarin vs DOACs: 3.5% vs 1.0% a yearIntracranial bleeding with warfarin vs DOACs: 1.1% vs 0.2% a year |
AMI, acute myocardial infarction; DOACs, direct oral anticoagulants; HR, hazard ratio; SE, systemic embolism; TIA, transient ischemic attack; VKA, vitamin K antagonist.
According to definition of International Society on Thrombosis and Hemostasis: fall in hemoglobin level ≥2g/dl, transfusion of ≥2 units of red blood cells, fatal bleeding or bleeding in a critical area (intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, or retroperitoneal).
Although there are no direct data on the use of DOACs in patients with nonvalvular AF, evidence suggests that they are a safe and effective option for patients with active cancer.35 The choice of agent will depend on clinical considerations applicable to routine usage (eg, age, weight, renal function, increased bleeding risk in patients with unoperated digestive and genitourinary tract tumors, and need for concomitant antiplatelet treatment) and the risk of interactions with cancer drugs (Table 5).35,50 As is the case for patients without cancer, dose adjustments based on renal function will reduce the risk of an embolic event due to underdosing.35,51,52 In patients with cancer requiring an invasive procedure or surgery, DOACs should be administered following the same criteria as for patients without cancer.53
Interactions between cancer drugs and anticoagulants
| Vitamin K antagonists | ||
|---|---|---|
| AcenocumarolWarfarin | Capecitabine, etoposide+carboplatin, 5–fluorouracil, ifosfamide, imatinib, paclitaxel, tamoxifen, dabrafenib, ivosidenib | Contraindicated/not recommended |
| Bicalutamide, carbozantinib, carboplatin, ceritinib, cyclophosphamide, cytarabine, cisplatin, dasatinib, doxorubicin, erlotinib, etoposide, gefitinib, ibritumomab, ibrutinib, imatinib, VEGF/VEGFR inhibitors, interferons, ipilimumab, irinotecan, methotrexate, nintedanib, obinutuzumab, procarbazine, regorafenib, romidepsin, rucaparib, sorafenib, sunitinib, tegafur, thiotepa, vorinostat | These drugs potentiate anticoagulant effect | |
| Mercaptopurine, mitotane, nilotinib | These drugs attenuate anticoagulant effect | |
| Low–molecular-weight heparins | ||
|---|---|---|
| Low–molecular-weight heparins | Capecitabine | Contraindicated/not recommended |
| Other cancer drugs | No interactions described | |
| Direct oral anticoagulants | ||
|---|---|---|
| Apixaban | Abiraterone, aprepitant, crizotinib, doxorubicin, enzalutamide, idelasilib, imatinib, sunitinib, vandetanib, vinblastine | Contraindicated/not recommended |
| • Axitinib, ciclosporin, dexamethasone, lapatinib, nilotinib, tacrolimus, tamoxifen• Paclitaxel, pazopanib, prednisone, sirolimus, temsirolimus, vemurafenib• Anastrozole, bicalutamide, cyclophosphamide, dasatinib, docetaxel, etoposide, idarubicin, ifosfamide, lomustine, vincristine, vinorelbine• Dasatinib, ibritumomab, nintedanib, obinutuzumab | Caution required in polymedicated patients and patients with ≥ 2 bleeding risk factors | |
| • Alkylating agents: bendamustine, busulfan, carmustine, chlorambucil, platinum complexes, dacarbazine, lomustine, melphalan, procarbazine, temozolomide• Antimetabolites: methotrexate• Cytotoxic antibiotics: bleomycin, daunorubicin, mitomycin, mitoxantrone• Monoclonal antibodies: alemtuzumab, bevacizumab, brentuximab, cetuximab, ipilimumab, nivolumab, rituximab, trastuzumab• Camptothecins: irinotecan, topotecan• Erlotinib, everolimus, flutamide, ibrutinib, letrozole, leuprolide, raloxifene | No relevant interactions expected | |
| Rivaroxaban | • Abiraterone, aprepitant, crizotinib, doxorubicin, enzalutamide, idelasilib, imatinib, sunitinib, vandetanib, vinblastine | Contraindicated/not recommended |
| • Paclitaxel, pazopanib, prednisone, sirolimus, temsirolimus, vemurafenib• Anastrozole, bicalutamide, cyclophosphamide, dasatinib, docetaxel, etoposide, idarubicin, ifosfamide, lomustine, vincristine, vinorelbine | Caution required in polymedicated patients and patients with ≥ 2 bleeding risk factors | |
| • Crizotinib, imatinib, ribociclib | Avoid in patients with advanced kidney failure | |
| • Dabrafenib, ivosidenib | These drugs reduce plasma concentrations of rivaroxaban | |
| • Alkylating agents: bendamustine, busulfan, carmustine, chlorambucil, platinum complexes, dacarbazine, lomustine, melphalan, procarbazine, temozolomide• Antimetabolites: methotrexate• Cytotoxic antibiotics: bleomycin, daunorubicin, mitomycin, mitoxantrone• Monoclonal antibodies: alemtuzumab, bevacizumab, brentuximab, cetuximab, ipilimumab, nivolumab, rituximab, trastuzumab• Camptothecins: irinotecan, topotecan• Erlotinib, everolimus, flutamide, ibrutinib, letrozole, leuprolide, ponatinib, raloxifene | No relevant interactions expected | |
| Edoxaban | • Abiraterone, aprepitant, crizotinib, doxorubicin, enzalutamide, idelasilib, imatinib, sunitinib, vandetanib, vinblastine | Contraindicated/not recommended |
| • Axitinib, crizotinib, lapatinib, ceritinib, ponatinib, sunitinib, vandetanib | Use with caution | |
| • Alkylating agents: bendamustine, busulfan, carmustine, chlorambucil, platinum complexes, dacarbazine, lomustine, melphalan, procarbazine, temozolomide• Antimetabolites: methotrexate• Cytotoxic antibiotics: bleomycin, daunorubicin, mitomycin, mitoxantrone• Monoclonal antibodies: alemtuzumab, bevacizumab, brentuximab, cetuximab, ipilimumab, nivolumab, rituximab, trastuzumab• Camptothecins: irinotecan, topotecan• Erlotinib, everolimus, flutamide, ibrutinib, letrozole, leuprolide, raloxifene• Vinca alkaloids: vincristine, vinorelbine• Alkylating agents: cyclophosphamide, ifosfamide, lomustine, melphalan• mTOR inhibitors: sirolimus, temsirolimus• Taxanes: docetaxel, paclitaxel• Anastrozole, bicalutamide, dasatinib, etoposide, idarubicin, pazopanib, prednisone, vemurafenib | No relevant interactions expected | |
| Dabigatran | • Abiraterone, crizotinib, ciclosporin, doxorubicin, enzalutamide, ibrutinib, imatinib, lapatinib, lenvatinib, sunitinib, tacrolimus, vandetanib, vemurafenib, vinblastine | Contraindicated/not recommended |
| • Axitinib, dexamethasone, lapatinib, nilotinib, ponatinib, tamoxifen, crizotinib | Caution required in polymedicated patients and patients with ≥ 2 bleeding risk factors | |
| • Vinca alkaloids: vincristine, vinorelbine• Alkylating agents: bendamustine, busulfan, carmustine, cyclophosphamide, chlorambucil, platinum complexes, dacarbazine, ifosfamide, lomustine, melphalan, procarbazine, temozolomide• Adenosine, pyrimidine, and purine analogs• Androgen receptor antagonists: bicalutamide, flutamide• Cytotoxic antibiotics: bleomycin, daunorubicin, idarubicin, mitomycin, mitoxantrone• Monoclonal antibodies: alemtuzumab, bevacizumab, brentuximab, cetuximab, ipilimumab, nivolumab, rituximab, trastuzumab• Antimetabolites: methotrexate• Camptothecins: irinotecan, topotecan• Aromatase inhibitors: anastrozole, letrozole• mTOR inhibitors: everolimus, sirolimus, temsirolimus• Taxanes: docetaxel, paclitaxel• Dasatinib, erlotinib, etoposide, leuprolide, pazopanib, prednisone, raloxifene, vemurafenib | No relevant interactions expected | |
VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Clinical practice guidelines recommend percutaneous left atrial appendage closure as a safe and effective alternative to anticoagulant treatment for patients with high embolic risk and contraindications for long-term anticoagulation.8,54 As patients with cancer have an increased risk of bleeding, percutaneous left atrial appendage closure could be considered by the C-O-H team as an option for patients with nonvalvular AF, patients with a contraindication to anticoagulant therapy, and those with a life expectancy of more than 1 year.55 The choice of antiplatelet or anticoagulation therapy to use for the months immediately following the procedure should be made in advance.8
AF PreventionOne of the most important goals of a C-O-H team is to stratify baseline risk of CV complications due to cancer therapy and to establish appropriate preventive measures. In patients at risk of AF, the strategy should be to prevent CV and non-CV triggers in order to minimize the proarrhythmic effects associated with cancer treatments.1,3,6,8
AF prevention for patients at high CV riskFirst and foremost, patients with cancer should be advised to follow the same general CV prevention strategies as those without cancer,8,24,56 in particular, smoking and alcohol cessation, moderate daily physical exercise, and weight control.57 A sustained weight loss of 10% or more has been associated with a 6-fold increased likelihood of arrhythmia-free survival in patients with AF, while weight fluctuations of over 5% have been found to double the risk of AF recurrence.58 This risk can be reduced through optimal blood pressure control,59 which is occasionally altered by cancer therapy, and treatment with continuous positive airway pressure in patients with sleep apnea.8 Multidisciplinary CV risk management programs are crucial for improving treatment adherence and reducing thromboembolic complications in this setting.60
AF prevention for patients with structural heart diseaseCancer, heart failure, and ischemic heart disease are closely related both to each other and to AF.1,3,8,61,62 Patients with AF are at increased risk of heart failure and ischemic heart disease and both these conditions increase the risk of AF.63,64 The risk is even greater in patients with active cancer due to the toxicity of therapy and the presence of comorbidities.1 General prevention and treatment recommendations applicable to patients without cancer also apply here, although there are a number of special considerations that must be addressed (see Table 6 and the section on special circumstances).8
Recommendations for treating atrial fibrillation in patients with cancer and heart disease
| 1. Treat triggers (eg, fever, pain, infection, hypoxemia) and attempt to control heart rate before heart rhythm, as sinusal reversion sometimes occurs spontaneously and this strategy also reduces the risk of early recurrence |
| 2. Before deciding on a heart rhythm control strategy, check potential interactions between antiarrhythmics and cancer drugs ( and Table 5) |
| 3. Early ultrasound assessment of patients with cancer, AF, and heart failure can help identify potential triggers, such as pulmonary thromboembolism, pericardial effusion and cardiac tamponade, valve disease, tumor invasion, and endocarditis |
| 4. When ventricular dysfunction is observed in patients with rapid-onset AF, repeat echocardiographic assessment once heart rate is controlled to prevent overdiagnosis of ventricular dysfunction due to cardiotoxic effects |
| 5. Because heart failure is a major risk factor for embolism, anticoagulation should always be considered, regardless of other factors on the CHA2DS2-VASC scale, even age |
| 6. In patients being treated with checkpoint inhibitors, AF and heart failure could be the first manifestations of immune-mediated myocarditis. These patients should therefore be referred for priority cardio-oncology evaluation |
| 7. Check indications for antiplatelet treatment in patients who need anticoagulation |
AF, atrial fibrillation; CHA2DS2-VASc, acronym for congestive heart failure; hypertension, age ≥ 75 y (double score), diabetes mellitus, stroke (double score) vascular disease, age 65-74 y and sex (female).
Postoperative AF is a common, well-established complication of oncologic surgery, with an incidence of around 20%.6 It is more common in thoracic surgery and in obese patients, patients older than 65 years, and patients with pre-existing CV or bronchial disease. Clinical management is complicated by the increased risk of bleeding associated with surgery. Prophylaxis with metoprolol or losartan has been found to reduce the incidence of postoperative AF in patients with elevated N-terminal pro-brain natriuretic peptide after lung cancer surgery.17
The role of ultrasound in assessing AF riskUltrasound assessment of patients receiving active cancer therapy improves stratification of AF risk (higher in patients with left atrium dilation [volume >34mL/m2], left ventricular systolic dysfunction, significant pulmonary hypertension, moderate to severe valve disease, and pericardial effusion) and also provides important information on the likelihood of maintaining sinus rhythm in relation to heart disease.65
Special circumstances in patients with cancer and AFAntiplatelet therapyIschemic heart disease is one of the main indications for antiplatelet therapy and it often coexists with nonvalvular AF. Duration of combined anticoagulant and antiplatelet therapy should be limited in cancer patients with ischemic heart disease as it increases the risk of bleeding, particularly in patients with digestive, genitourinary tract, or central nervous system tumors (Figure 2).66,67 Triple therapy for at least 1 month can be considered in patients with acute coronary syndrome and can be extended up to 3 to 6 months for patients with high ischemic and low hemorrhagic risk. Clopidogrel is generally favored over other P2Y12 inhibitors in combination therapies as it has a lower bleeding risk. Rigorous monitoring of INR values (2-2.5) is needed in patients on VKA therapy, although target values are difficult to achieve during active cancer treatment, making DOACs a good alternative. Data from the PIONEER AF-PCI,68 REDUAL PCI,69 and AUGUSTUS70 trials support the safety of rivaroxaban, dabigatran, and apixaban as respective alternatives for dual therapy with clopidogrel after percutaneous coronary intervention (PCI). Oral anticoagulation alone can be continued for 1 year after acute coronary syndrome or elective PCI. Treatment duration should always be tailored to individual ischemic and bleeding risk (Figure 2).
Antiplatelet treatment for patients with active cancer and atrial fibrillation who need anticoagulation. High IR: history of stent thrombosis, multivessel disease or multiple stents, diabetes mellitus, acute coronary syndrome, PCI in bifurcation lesions or chronic occlusions; Increased BR in patients with cancer: HAS-BLED score >3, involvement of CNS or GU or GI tract, disseminated disease, previous bleeding, platelet count <50 000/μL, significant anemia, or need for transfusion or concomitant cancer treatment that increases BR. A, aspirin; BR, bleeding risk; C, clopidogrel; CNS, central nervous system; DOACs, direct oral anticoagulants; GI, gastrointestinal; GU, genitourinary; HAS-BLED, acronym for hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol; IR, ischemic risk.
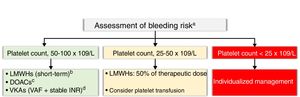
Thrombocytopenia (platelet count <100×109/L) is common in cancer. It is mainly caused by cancer treatments, but it can also be induced by other factors, such as bone marrow invasion by tumor cells, hypersplenism, and immune- or nonimmune-mediated platelet destruction. Anticoagulant therapy in patients with active cancer, AF, and thrombocytopenia is complicated, as thromboembolic risk does not decrease despite the high bleeding risk.71 VKA therapy is also complicated because of unpredictable treatment responses and the exclusion of patients with platelet counts of less than100×109/L (RELY, ARISTOTLE, and ENGAGE AF-TIMI 38) or90×109/L (ROCKET AF) from major clinical trials.8,35
There are no absolute contraindications to anticoagulant therapy in patients with CHA2DS2-VASc scores of 2 or higher or platelet counts of over50×109/L. VKA therapy can be contemplated in valvular AF patients with stable INR values in the absence of significant DDIs. LMWHs can be considered as a short-term alternative, and DOACs are an interesting option for nonvalvular AF, again in the absence of DDIs. Finally, low-dose LMWHs can be considered in patients with high thrombotic risk (mechanical heart valves, rheumatic mitral stenosis, history of systemic embolism, and CHA2SD2-VASc score ≥3) and a platelet count of 25-50×109/L. Treatment for patients with a platelet count of less than25×109/L should be decided on a case-by-case basis by the C-O-H team (Figure 3).
Anticoagulation in patients with cancer, atrial fibrillation, and thrombocytopenia. aHAS-BLED score >3, individualized decision; for patients with mild thrombocytopenia and a high bleeding risk, the option of atrial appendage closure should be discussed by the cardio-onco-hematology team. bIf transient thrombocytopenia is expected. Check interactions. cDOACs are an alternative to LMWHs in the absence of contraindications and when there is a preference for oral administration. Check interactions. Use with caution in patients with gastrointestinal or genitourinary tract tumors with a nephrostomy tube. There is no evidence regarding patients with a glomerular filtration rate of <15mL/min. Contrindicated for valvular atrial fibrillation. dVKAs for patients with valvular AF and stable INR values. Check interactions. AF, atrial fibrillation; DOACs, direct oral anticoagulants, HAS-BLED, acronym for hypertension, abnormal renal and liver function, stroke, bleeding, labile inr, elderly, drugs or alcohol; IR, ischemic risk; CNS, central nervous systemINR, international normalized ratio; LMWH, low–molecular-weight heparins; VAF, valvular atrial fibrillation; VKAs, vitamin K antagonists.
Chronic kidney disease increases both ischemic and bleeding risk. DOACs have been found to be safer and more efficacious than VKAs in patients with a glomerular filtration rate (GFR) of over 30mL/min. No clinical trials, however, have evaluated the use of anticoagulants in patients with a GFR of less than 15mL/min or in patients undergoing renal replacement therapy.35,52 Apixaban has the lowest renal elimination rate of all DOACs. Compared with VKAs, it is more likely to reduce bleeding risk at lower GFR categories without losing its protective effect against thrombosis.35 Dabigatran, by contrast, has the highest renal elimination rate and loses its protective effect against bleeding in patients with a GFR of less than 50mL/min. DOAC treatment must be individualized in patients with AF, active cancer, and kidney failure and it is essential to carefully monitor clinical changes such as dehydration, intercurrent infections, sepsis, hypotension, and cancer drug-induced nephrotoxicity.3
Valvular AFDOACs are currently contraindicated in patients with valvular AF and the standard recommendation is to maintain VKA therapy with strict INR monitoring.8 LMWHs can be used as a temporary alternative in the event of serious DDIs or oral intolerance during cancer treatment. In such cases, doses must be adjusted to patient weight and renal function and, where possible, anti-Xa activity should be monitored (target, 0.5-1 U/mL).72
FrailtyWith an increasingly old population, the concept of frailty is gaining traction due to its prognostic impact on different clinical scenarios within the setting of CV disease.73 The best scale that has been validated for use in clinical practice is the Comprehensive Geriatric Assessment, which is a multidisciplinary tool for assessing clinical, functional, cognitive, social, and nutritional status. Frailty is associated with a worse prognosis in patients who undergo oncologic surgery, chemotherapy, or radiotherapy,74 and treatment is further complicated by the presence of AF, which is associated with an increased risk of thrombosis, bleeding, and heart failure. This increased risk is due to the tumor and anticoagulant therapy as well as metabolic changes in patients with multiple comorbidities, DDIs, falls due to frailty, and suboptimal treatment adherence in patients with cognitive impairment or poor social support.3,34,73 Frailty must be managed from a multidisciplinary approach. Physical rehabilitation programs, treatment adjustments in the case of polypharmacy, and nutritional, psychological, and social support are all valuable interventions.73
Conclusions and final recommendations- •
CV risk factors and comorbidities associated with AF must be identified and treated to reduce the incidence of AF in patients with cancer.
- •
Neither AF (prevalent or past) nor AF risk factors are a contraindication for OHT, but they do require multidisciplinary management by a C-O-H team.
- •
Patients with pre-existing AF should be assessed by a C-O-H team to optimize AF treatment during active cancer therapy.
- •
Patients newly diagnosed with AF should be evaluated by the C-O-H team without delay to decide on the best treatment strategy and to prevent unnecessary interruptions of cancer therapy.
- •
Heart rate rather than rhythm control strategies are preferable in most patients undergoing active cancer therapy, as rhythm control strategies have little success during this period.
- •
In the absence of specific scales for stratifying thromboembolic risk associated with AF in patients with active cancer, the choice of antithrombotic treatment should be guided by CHA2DS2-VASc scores. Treatment must be individualized with consideration of bleeding risk.
- •
Treatment with VKAs is generally problematic in patients receiving active cancer therapy due to the difficulty of ensuring stable INR values and the risk of DDIs.
- •
For patients with mechanical heart valves or moderate to severe rheumatic mitral stenosis, the decision to maintain VKA treatment or switch to an LWMH should be taken on a case-by-case basis.
- •
Although there are no direct data on the use of DOACs in patients with nonvalvular AF, these anticoagulants are safe and effective and, in our opinion, should be the treatment of choice in this setting.
- •
The choice of DOAC should be tailored to each patient according to potential DDIs and the presence of comorbid conditions.
- •
Efforts are needed to promote the development of specific scales for accurately stratifying thromboembolic and bleeding risk in patients with AF and active cancer.
- •
Randomized clinical trials are also needed to confirm the safety and efficacy of DOACs in patients with AF and active cancer.
None declared.