Since the first transcatheter aortic valve implant (TAVI) was performed by Cribier et al. in 2002,1 the technique and the devices used have undergone such development that this intervention, initially designed for inoperable patients,2 has changed the paradigm of aortic stenosis,3 and it now represents a safe and effective option for low-risk patients, according to the results of the PARTNER 3 trial.4
The choice of device for TAVI is made based on the operator's experience, the hospital's systems, and the most suitable option for the patient. In our setting, there is an increasing range of self-expanding valves: Evolut R/Pro (Medtronic, USA), ACURATE neo (Boston Scientific, Switzerland), Portico (Abbott, USA) and Allegra (NVT, Germany). However, until recently, the only balloon-expandable system option has been SAPIEN 3 (Edward Lifesciences, USA), which demonstrated clinical outcomes superior to those with surgical valve replacement in many situations, and better than those with self-expanding valves in unselected patients.5
Innovation in this field has allowed the development of the balloon-expandable Myval prosthetic valve (Meril Life Sciences Pvt Ltd, India), which obtained the European Community (CE) mark following the Myval-1 study,6 conducted in 30 patients with intermediate-high risk: it demonstrated the safety and efficacy of the valve, with no reported paravalvular leak, significant aortic regurgitation, or need for permanent pacing after implantation, with a 12-month survival of 86.67%. These data are similar to the percutaneous arm of the PARTNER-3 trial, although more data are still needed. The structure of the valve is similar to that of SAPIEN 3 (figure 1a), but it has certain advantages. One of the main developments is that the available sizes include valve diameters of 20, 23, 26 and 29mm, like the SAPIEN 3 system, but also 21.5, 24.5, and 27.5 mm, which limits the extent of aortic annulus overexpansion and reduces its risk of rupture, one of the main concerns when implanting a balloon-expandable prosthesis. Diameters of 30.5 and 32mm are soon to be added, covering a wider range of sizes. In addition, the system is inserted via a 14-Fr expandable sheath, meaning a lower entry profile than with other devices.
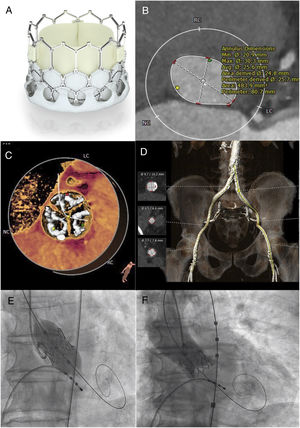
A: Myval prosthesis. B: measurement of the aortic annulus using computed tomography (patient 1). C: distribution of calcium on valve leaflets (patient 1). D: aortoiliac-femoral axis (patient 1). E: angiographic image at implantation of Myval aortic prosthesis. F: final angiogram with contrast after Myval implantation.
Before implantation, it is recommended to perform predilatation with a Mammoth balloon (Meril Life Sciences Pvt Ltd, India), compatible with a 0.035-inch conventional guidewire. The valve is crimped on its over-the-wire balloon catheter system (Navigator, Meril Life Sciences Pvt Ltd, India), which has a high-flex design, to reduce trauma during arch navigation, reduce the risk of periprocedural stroke, and optimize its fit within the aorta. At the beginning of its inflation, the balloon has a dog-bone shape; its position is further stabilized as it is inflated, along with rapid ventricular pacing with a pacemaker. The valve is introduced already mounted on the balloon, unlike the SAPIEN 3 system, which is mounted on the balloon in the descending aorta.
We present the first 2 cases of Myval implantation in Spain. The first was in an 83-year-old man, in New York Heart Association (NYHA) functional class III due to severe aortic stenosis with a mean gradient of 78mmHg and aortic valvular area of 0.60 cm2, with preserved ejection fraction. His surgical risk calculated according to Euroscore II was 2.99%; surgery was ruled out due to his advanced age and low weight (57kg). Right femoral and radial arterial access was obtained and a temporary pacemaker was positioned via left femoral venous access. Preclosure was performed with 2 Proglide (Abbott Vascular, USA) and the 14-Fr sheath was introduced. Under rapid ventricular pacing, predilatation was performed with an 18-mm Mammoth balloon and, according to the dimensions and anatomical and calcium assessment obtained from computed tomography analysis (figure 1B-D), a 24.5-mm Myval was implanted (figure 1E), with trivial paravalvular leak (figure 1F).
The second was in a 79-year-old man, in NYHA class II secondary to severe aortic stenosis (mean gradient, 50mmHg; aortic valve area, 0.60 cm2; ejection fraction, 67%) with a Euroscore II of 1.55%. Surgery was ruled out due to severe lung disease. A 23-mm valve was implanted following the process described, which was unincidental and left minimal paravalvular regurgitation (). Both patients spent the first 24hours in the coronary care unit for hemodynamic monitoring and were then transferred to the cardiology ward, where echocardiography confirmed that their prosthetic valves were correctly positioned, with mild paravalvular leak in the first patient, and no signs of leak in the second patient, and mean gradients of 9 and 10mmHg, respectively. They were discharged at 4 and 5 days, respectively, without complications.
The development of the transcatheter Myval system demonstrates the importance of technical innovation in response to the growing demand for this intervention and calls from health professionals for maximum efficacy at minimum cost. Its added value lies in the smaller sheath size, which makes it a good alternative in patients with poor vascular access, and its greater range of sizes, which, as well as covering larger sizes, helps avoid overexpansion and reduces the risk of annular rupture.
Its efficacy must be demonstrated in the LANDMARK trial, which is currently in its initial phases.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.recesp.2020.01.025