The care of patients with acutely decompensated heart failure is being reshaped by the availability and understanding of several novel and emerging heart failure biomarkers. The gold standard biomarkers in heart failure are B–type natriuretic peptide and N-terminal pro-B-type natriuretic peptide, which play an important role in the diagnosis, prognosis, and management of acute decompensated heart failure. Novel biomarkers that are increasingly involved in the processes of myocardial injury, neurohormonal activation, and ventricular remodeling are showing promise in improving diagnosis and prognosis among patients with acute decompensated heart failure. These include midregional proatrial natriuretic peptide, soluble ST2, galectin–3, highly–sensitive troponin, and midregional proadrenomedullin. There has also been an emergence of biomarkers for evaluation of acute decompensated heart failure that assist in the differential diagnosis of dyspnea, such as procalcitonin (for identification of acute pneumonia), as well as markers that predict complications of acute decompensated heart failure, such as renal injury markers. In this article, we will review the pathophysiology and usefulness of established and emerging biomarkers for the clinical diagnosis, prognosis, and management of acute decompensated heart failure.
Keywords
Acutely decompensated heart failure (ADHF) is a common and heterogeneous condition that is difficult to diagnose and treat. Evaluation and correct recognition of ADHF in patients with dyspnea (the cardinal symptom of affected patients) may be challenging1; when there are doubts about the diagnosis, higher risk is observed. Additionally, delay in diagnosis of ADHF is associated with higher mortality.2 As a consequence, ADHF is not only morbid but is associated with significant health care expenditures. Improvement in the evaluation and management of the diagnosis is imperative, particularly with the rising incidence and prevalence of heart failure (HF) in the community.
While the basis of assessment for ADHF is (and always should be) standard history and physical examination, adjunctive testing to support clinical judgment has been shown to improve the accuracy of diagnosis and to aid prognostication and management. To be useful, such adjunctive testing should be rapidly available, easily interpretable, add to clinical variables and other objective tests, and should be cost–effective.3 In this regard, over the last decade, several biomarkers have emerged to aid in the diagnosis, risk stratification, and management of ADHF.
NATRIURETIC PEPTIDESAmong the biomarkers in ADHF, natriuretic peptides are the best studied and validated, representing the gold standard biomarker that serves as a comparison for all other markers. The utility of B–type natriuretic peptide (BNP) and its inactive form, N-terminal pro-B-type natriuretic peptide (NT-proBNP), is reflected in their incorporation into clinical practice guidelines for the diagnosis of HF, as published by the American College of Cardiology, the American Heart Association,4 the Heart Failure Society of America,5 and the European Society of Cardiology.6
Both atrial natriuretic peptide (ANP) and BNP are primarily produced in the myocytes of the atria and ventricles, respectively,7 and are produced in response to myocyte stretch from volume or pressure overload.8 While ANP is premade and stored in cytosolic granules within the cardiomyocyte, BNP is synthesized de novo when the need arises. Following a series of processing steps during synthesis, in both cases, a propeptide is produced as the penultimate form of ANP and BNP; following the effects of proteolytic enzymes, corin and furin, the propeptides (pro–ANP and NT–proBNP) are cleaved from mature ANP and BNP, and are released at the same time as the biologically active C–terminal hormonal natriuretic peptides. As will be discussed, amino–terminal propeptides are substantially equivalent to the measurement of the mature, biologically–active natriuretic peptides from which they were cleaved for diagnostic/prognostic application.
While ANP, BNP, and their amino–terminal propeptide equivalents are cleared passively by several organs, including the kidneys, following their release, ANP and BNP also bind to natriuretic peptide receptors, which results in the generation of cyclic guanosine monophosphate, and leads to a cascade of favorable biological responses that teleologically reflect a “response” to the deranged physiology of HF: due to activation of guanylate cyclase, both ANP and BNP trigger vasodilation, natriuresis, and diuresis. Additionally, both lead to a reduction in the effects of the renin–angiotensin–aldosterone system, reduce myocardial stiffness, and improve lusitropy. Beyond passive removal and receptor clearance, both ANP and BNP are also rapidly degraded by catalytic enzymes in circulation, notably including the enzyme neprilysin.
Advances in the understanding of natriuretic peptide biology have led to the recognition of high complexity in their biology. For reasons that are not entirely clear, as HF worsens, it is now known that a greater percentage of circulating “BNP” and “NT–proBNP” is actually uncleaved precursor peptide (proBNP); assays for BNP and NT–proBNP cannot resolve whether they are measuring free peptide or the precursor, because the peptide contains both regions recognized by the assays. Notably, proBNP does not have the same ability as BNP to trigger cyclic guanosine monophosphate; thus, patients with high levels of proBNP show an effect, called the “natriuretic peptide handicap”, in which, despite high levels of “BNP” they do not exhibit the effects of the mature, biologically active peptide.9 Additionally, through the effects of neprilysin and other enzymes, BNP circulates as a mixture of variably degraded fragments, with relatively little mature 32 amino acid BNP.10
Diagnostic EvaluationNatriuretic peptides are objective and reproducible, making them a potentially valuable tool in the evaluation of patients with suspected or proven HF. Several important studies have demonstrated the usefulness of BNP and NT–proBNP in conjunction with clinical judgment to diagnose or exclude ADHF.11–13 Moreover, both of these natriuretic peptides have been shown to be useful for diagnostic evaluation of ADHF in both HFrEF (HF with reduced ejection fraction) and HF with preserved ejection fraction (HFpEF),14 albeit with slightly reduced sensitivity in patients with HFpEF due to generally lower BNP and NT–proBNP levels among these patients.15
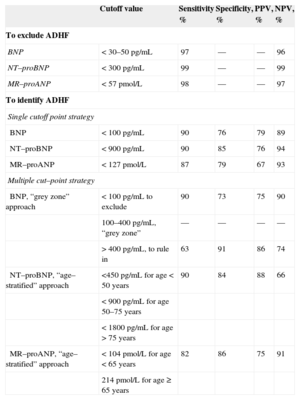
Suggested cutoffs for diagnostic use of BNP and NT–proBNP are depicted in Table 1. In the Breathing Not Properly study,15 BNP concentrations were higher in patients judged to have ADHF compared with those who did not (110 ± 225 pg/mL vs 675 ± 450 pg/mL) and differed significantly as a function of New York Heart Association HF severity (P < .001). A BNP concentration above 100 pg/mL had a sensitivity of 90%, specificity 76%, and accuracy 83% for differentiating HF from other causes of dyspnea, and when compared with history, physical examination, laboratory values, and chest X–ray findings, BNP was the single best predictor of a final diagnosis of HF (area under the curve [AUC] = 0.91; 95% confidence interval [95%CI], 0.90-0.93; P < .001).11
Suggested Natriuretic Peptide Cutpoints in Acute Decompensated Heart Failure
| Cutoff value | Sensitivity % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|---|
| To exclude ADHF | |||||
| BNP | < 30–50 pg/mL | 97 | — | — | 96 |
| NT–proBNP | < 300 pg/mL | 99 | — | — | 99 |
| MR–proANP | < 57 pmol/L | 98 | — | — | 97 |
| To identify ADHF | |||||
| Single cutoff point strategy | |||||
| BNP | < 100 pg/mL | 90 | 76 | 79 | 89 |
| NT–proBNP | < 900 pg/mL | 90 | 85 | 76 | 94 |
| MR–proANP | < 127 pmol/L | 87 | 79 | 67 | 93 |
| Multiple cut–point strategy | |||||
| BNP, “grey zone” approach | < 100 pg/mL to exclude | 90 | 73 | 75 | 90 |
| 100–400 pg/mL, “grey zone” | — | — | — | — | |
| > 400 pg/mL, to rule in | 63 | 91 | 86 | 74 | |
| NT–proBNP, “age–stratified” approach | <450 pg/mL for age < 50 years | 90 | 84 | 88 | 66 |
| < 900 pg/mL for age 50–75 years | |||||
| < 1800 pg/mL for age > 75 years | |||||
| MR–proANP, “age–stratified” approach | < 104 pmol/L for age < 65 years | 82 | 86 | 75 | 91 |
| 214 pmol/L for age ≥ 65 years | |||||
ADHF, acute decompensated heart failure; BNP, B–type natriuretic peptide; MR–proANP, midregional proatrial natriuretic peptide; MR–proANP: midregional proatrial; NPV, negative predictive value; NT–proBNP, N-terminal pro-B-type natriuretic peptide; PPV, positive predictive value.
NT–proBNP has also been shown to have similarly powerful clinical properties. In the PRIDE study,12 NT–proBNP at age–specific cutpoints was highly sensitive and specific for the diagnosis of ADHF. Not only was NT–proBNP the strongest independent predictor of a final diagnosis of ADHF (odds ratio [OR] = 44; P < .001), its diagnostic usefulness when combined with clinical judgment was superior to those of both NT–proBNP testing and clinical judgment alone. Subsequent analysis in the International Collaborative of NT–proBNP study showed that serum concentrations of NT–proBNP correlated significantly with ADHF symptoms, and confirmed the importance of age stratification for diagnostic accuracy: age–specific cutpoints of 450 pg/mL, 900 pg/mL, and 1800 pg/mL for ages < 50 years, 50 to 75 years, and > 75 years, respectively, yielded 90% sensitivity and 84% specificity for ADHF, and an age–independent cutoff of less than 300 pg/mL had a 98% negative predictive value to exclude ADHF.13
The usefulness of natriuretic peptides for cost–effective care in acute dyspnea has been evaluated in several analyses. NT–proBNP was examined in the randomized IMPROVE–CHF study,16 which showed significant improvement in cost expenditures ($6129–US $5180 per patient; P = .023) and superior outcomes associated with the addition of NT–proBNP to the diagnostic evaluation of patients in the emergency department setting.16 Similar findings were reported by Mueller et al in the BASEL study,17 in which randomization to a diagnostic strategy including BNP was associated with less intensive care unit admission and lower expenditures compared with standard evaluation. Using decision analytic framework analyses, Siebert et al demonstrated substantial cost–effectiveness associated with the use of NT–proBNP in the PRIDE study.18 In this analysis, NT–proBNP–guided assessment was associated with a 1.6% relative reduction of serious adverse event risk and a 9.4% reduction in costs, translating into savings of $474 per patient compared with standard clinical assessment. In a sensitivity analysis of mortality, NT-proBNP testing was associated with a 1.0% relative reduction in postdischarge mortality. Notably, optimal use of NT–proBNP guidance could potentially reduce the use of in–patient echocardiography by up to 58%, prevent 13% of initial hospitalizations, and reduce hospital days by 12%.
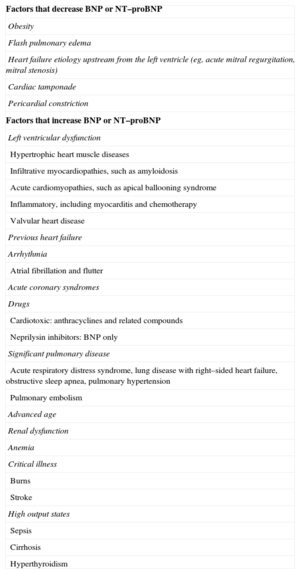
While BNP and NT–proBNP are useful tools for the diagnostic evaluation of patients with suspected or proven ADHF, there are some caveats regarding their use. In approximately 20% of patients, values for BNP or NT–proBNP fall into an intermediate or “grey” zone, where they may neither diagnose nor exclude ADHF.19 In these circumstances, clinical judgment may assist in identifying the correct diagnosis. In this regard, natriuretic peptide assays should never be used in isolation: they are adjunctive to clinical judgment, and while they may be extremely helpful in assisting clinical suspicion for ADHF, they are particularly useful in circumstances of clinical indecision.20 Both biomarkers are influenced by certain demographics and disease states (Table 2), including other cardiac and noncardiac causes. Much as with any diagnostic tool, the clinician must understand the differential diagnosis of an elevated BNP or NT–proBNP, in order to correctly interpret results.21
Factors Influencing Natriuretic Peptide Concentrations
| Factors that decrease BNP or NT–proBNP |
| Obesity |
| Flash pulmonary edema |
| Heart failure etiology upstream from the left ventricle (eg, acute mitral regurgitation, mitral stenosis) |
| Cardiac tamponade |
| Pericardial constriction |
| Factors that increase BNP or NT–proBNP |
| Left ventricular dysfunction |
| Hypertrophic heart muscle diseases |
| Infiltrative myocardiopathies, such as amyloidosis |
| Acute cardiomyopathies, such as apical ballooning syndrome |
| Inflammatory, including myocarditis and chemotherapy |
| Valvular heart disease |
| Previous heart failure |
| Arrhythmia |
| Atrial fibrillation and flutter |
| Acute coronary syndromes |
| Drugs |
| Cardiotoxic: anthracyclines and related compounds |
| Neprilysin inhibitors: BNP only |
| Significant pulmonary disease |
| Acute respiratory distress syndrome, lung disease with right–sided heart failure, obstructive sleep apnea, pulmonary hypertension |
| Pulmonary embolism |
| Advanced age |
| Renal dysfunction |
| Anemia |
| Critical illness |
| Burns |
| Stroke |
| High output states |
| Sepsis |
| Cirrhosis |
| Hyperthyroidism |
BNP, B–type natriuretic peptide; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
In certain circumstances, results for BNP or NT–proBNP may be unexpectedly high. While many of these situations are explained by the presence of underlying structural cardiopulmonary diseases, it is imperative to remember that not all elevations of either peptide are related to acute left HF; indeed, when related to right ventricular strain, marked elevation of BNP or NT–proBNP may be seen in patients with acute pulmonary thromboembolism,22 a diagnosis with considerably different management steps than ADHF on the basis of left HF. Beyond these extreme examples, it is worth emphasizing more subtle situations associated with modest elevation in BNP or NT–proBNP. Among these are anemia, advancing age, and renal failure.
With respect to age, both BNP and NT–proBNP are similarly affected, with higher values for both peptides seen in elderly patients; this is related to an accumulation of structural heart disease, including diastolic noncompliance with aging. This situation can be somewhat mitigated by the use of age–adjusted cutoffs for NT–proBNP.
Given the equal dependence of BNP or NT–proBNP on renal function for their clearance,23 more advanced stages of renal dysfunction show higher values of both peptides. This is partly related to reduced clearance, but importantly also reflects the prevalent cardiac abnormalities in patients with renal dysfunction, as well as expanded circulating volume related to reduced glomerular filtration. Strategies to improve diagnostic accuracy in renal failure include the use of alternative cutoffs (Table 1) or, in the case of NT–proBNP, use of age–adjusted cutpoints, as discussed above.
A soon–to–emerge situation associated with higher than expected BNP values will be treatment with drugs exerting neprilysin inhibition. In the recent landmark PARADIGM–HF trial,24 use of LCZ696 (an agent containing a neprilysin inhibitor) was superior to enalapril for the care of patients with chronic HF.24 Treatment with LCZ696 resulted in significantly higher BNP concentrations, part of the therapeutic effect of the drug25; NT–proBNP is not a substrate for neprilysin, and as such did not spuriously rise. As the use of LCZ696 is likely to substantially change the landscape of HF care, clinicians are warned that the effect of the drug on BNP concentrations is not quantifiable, and consequently interpretation of BNP concentrations in patients so–treated may be extremely challenging. Proper interpretation of NT–proBNP is not clouded by use of neprilysin inhibition.
Unexpectedly lower concentrations of BNP and NT–proBNP in the setting of ADHF do occur, and are most often seen in states of HF with lower wall stress (eg, HFpEF), as well as HF involving right–sided cardiac structures (eg, isolated tricuspid regurgitation) due to less myocardium in these chambers. Additionally, very importantly, obesity is associated with a 15%–20% false negative rate for BNP and NT–proBNP in the setting of ADHF.26 This is thought to be due to reduction in the release of these peptides in the setting of higher body mass index. While both peptides are typically lower in the most overweight individuals, they are not “normal” compared with a healthy individual, and when elevated, both peptides retain their prognostic meaning.27
Lastly, of late, midregional proatrial natriuretic peptide (MR–proANP) has emerged as a promising biomarker for diagnosis or exclusion of ADHF; MR–proANP was found to be noninferior to BNP for ADHF diagnosis in the BACH trial.28 That same study also showed that MR–proANP improved diagnostic accuracy in the presence of a grey zone BNP value (between 100 pg/mL and 500 pg/mL). In a subsequent analysis of the PRIDE study, MR–proANP concentrations were higher in patients with ADHF (median 329pmol/L vs 58pmol/L; P < .001) and the marker was found to be an independent predictor of ADHF (OR = 4.34; 95%CI, 2.11-8.92; P < .001), curiously additive to NT–proBNP for this application.29 Suggested cutoffs for the use of MR–proANP are depicted in Table 1. In addition to improving diagnosis, MR–proANP holds promise for prognostication beyond NT–proBNP and clinical risk factors in net reclassification analyses, and changes in MR–proANP over time appear to be predictive of mortality.30 In contrast to the findings from BACH, however, MR–proANP had a lower AUC than NT–proBNP under the receiver operating characteristic curve for diagnosis of ADHF (AUC = 0.90, 95%CI = 0.87–0.93; P < .001 compared with AUC = 0.94; 95%CI, 0.92–0.96; P < .001; P = .001 for difference). Whether MR–proANP will be used as a stand–alone diagnostic tool for ADHF remains uncertain.
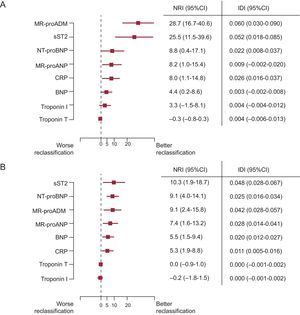
Prognosis and ManagementBeyond their value for diagnostic evaluation of ADHF, natriuretic peptides have individually and collectively been shown in many studies to also be useful in stratifying the risk for rehospitalization and/or death in patients with this diagnosis.1,13,31 For example, in one study, median NT–proBNP concentrations were higher in nonsurvivors at 76 days of follow-up (median, 10 426 [interquartile range, 5611-23 818] pg/ml) compared with survivors (4873 pg/mL [2204-10 897]; P < .001 for difference); in this analysis NT–proBNP > 5180 pg/mL was strongly associated with short–term mortality (OR = 5.2; 95%CI = 2.2–8.1). Importantly, the risk data imparted from knowledge of either BNP or NT–proBNP appears to be substantially additive to clinical variables, allowing for significant reclassification of risk beyond clinical information (Figures 1A and B).32 As with the diagnostic application of MR–proANP, it appears that the prognostic information imparted from measurement of this peptide is additive to that of NT–proBNP.29
Reclassification of risk added by biomarkers beyond a clinical model for 30-day mortality (A) and 1-year mortality (B). 95%CI, 95% confidence interval; BNP, B-type natriuretic peptide; IDI, integrated discrimination improvement; MR-proADM, midregional proadrenomedullin; MR-proANP, midregional proatrial natriuretic peptide; NRI, net reclassification improvement; NT-proBNP, N-terminal pro-B-type natriuretic peptide. Reproduced with permission from Lassaus et al.32
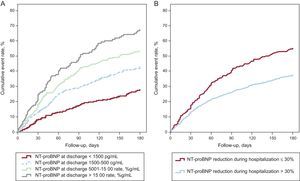
While a baseline natriuretic peptide value is useful for prognostication, it is unambiguously established that variations in BNP or NT–proBNP over the course of hospitalization and predischarge very strongly predict adverse outcomes; inadequate reduction or rise following treatment for ADHF is linked to risk for rehospitalization or death. Most recently, Salah et al34 showed that both the percent reduction, as well as the final concentration of the marker achieved after ADHF therapy, independently predicted risk (Figure 2).33 This supports a strategy of in–hospital monitoring using BNP or NT–proBNP, generally reserving 2 measurements for evaluation: baseline for diagnosis and risk stratification, and a posttreatment measurement for assessment of therapy response.34
Risk for death or rehospitalization for acute decompensated heart failure in hospitalized patients with acute decompensated heart failure as a function of N-terminal pro-B-type natriuretic peptide concentration achieved at discharge (A) or whether a 30% change in N-terminal pro-B-type natriuretic peptide was achieved by discharge (B). NT-proBNP, N-terminal pro-B-type natriuretic peptide. Reproduced with permission from Salah et al.33
The role of natriuretic peptides to “guide” in–hospital therapy35 is a tantalizing potential application; conceptually, since therapies for ADHF cause a reduction in natriuretic peptide concentration that parallels the improved risk for hazard, it is logical to assume that a strategy that involves robust reduction in BNP or NT–proBNP as part of discharge criteria would be superior to a strategy without such biomarker guidance. This concept has yet to be evaluated prospectively; until such data are available, logic must dictate individual acceptance of this approach.
BEYOND THE NATRIURETIC PEPTIDES IN ACUTE DECOMPENSATED HEART FAILUREWhile BNP and NT–proBNP are of significant use for the evaluation and management of patients with ADHF, it is reasonable to expect that other biomarkers may add to their value. As noted above, for example, MR–proANP supplements the dominant diagnostic and prognostic information provided by BNP or NT–proBNP. Beyond the natriuretic peptides, however, newer biomarkers, unique relative to the natriuretic peptides, hold promise to provide “orthogonal” biological information.3,36 In general, these biomarkers either provide additive (or superior) prognostic cardiovascular information to the natriuretic peptides or inform other diagnoses that commonly occur in patients with ADHF, such as respiratory infection or renal dysfunction.
Soluble ST2After the natriuretic peptides, soluble ST2 (sST2) is among the most important new biomarkers for prognostic information in HF syndromes, providing powerful prognostic information. Soluble ST2 is a protein found to be upregulated in states of mechanical strain in cardiac myocytes37 and has subsequently been shown to play an important role in myocardial hypertrophy and fibrosis. This protein is also released by endothelial cells, and may be involved in the development of atherosclerosis and arterial hypertension.38,39
Early data supporting sST2 measurement came from the PRIDE study.40 In this analysis of 593 patients with acute dyspnea, sST2 concentrations were higher among those with ADHF, but NT–proBNP outperformed sST2 for ADHF diagnosis (AUC = 0.94 vs 0.80; P < .001 for difference). However, concentrations of sST2 were highly associated with HF symptom severity and were nearly linear with risk for short– and longer–term mortality; data from PRIDE show that sST2 concentrations were the strongest predictor of mortality at 30 days, 1 year, and 4 years, superior to NT–proBNP, and any other biomarker examined in the study, including galectin–3.40 Shah et al29 showed that concentrations of sST2 in patients with available echocardiography data from PRIDE were correlated with a broad variety of cardiovascular structural and functional abnormalities, including a more adversely remodeled left ventricle, lower left ventricular ejection fraction, worse diastolic compliance, and higher pulmonary artery pressures.
Using a pooled analysis, Rehman et al41 subsequently extended the understanding of sST2 in ADHF. They again found that values of sST2 correlated with the severity of HF, finding sST2 to represent a powerful predictor of mortality in patients with both HFrEF and HFpEF; indeed, among patients with HFpEF, Manzano–Fernández et al42 subsequently showed sST2 to be superior to NT–proBNP for prognosis. Relative to multiple other biomarkers, Lassus et al32 recently showed sST2 to provide the most consistently powerful reclassification beyond clinical variables for 1–year mortality after presentation with ADHF (Figures 1A and B).32
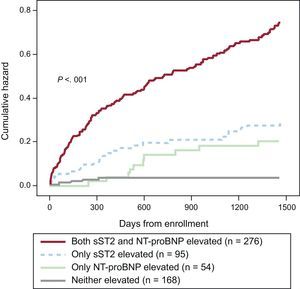
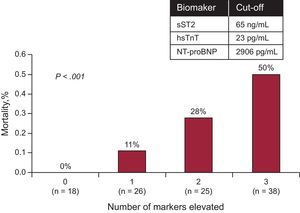
As noted, sST2 frequently provides prognostic information that supersedes that of BNP or NT–proBNP; however, given that the information provided by sST2 is fundamentally different than that of the natriuretic peptides, the 2 biomarkers provide additive/multiplicative prognostic information (Figure 3). Besides natriuretic peptides, the prognostic information from sST2 appears to be additive to highly sensitive troponin (hsTn) as well: in an analysis of 107 patients with ADHF, Pascual–Figal et al43 showed that sST2, high-sensitivity troponin T (hsTnT), and NT–proBNP all provided unique prognostic information for death over a 2 year period. Concentrations of sST2 [per 10 ng/mL, hazard ratio [HR] = 1.09; 95%CI, 1.04–1.13; P < .001], hsTnT (per 0.1 ng/mL, HR = 1.16; 95%CI, 1.09-1.24; P < .001), and NT-proBNP (per 100 pg/mL, HR = 1.01; 95%CI, 1.003-1.01; P < .001) were each predictive of a higher risk of death. In bootstrapped models, each biomarker retained independent predictive value for mortality; patients with all 3 biomarkers below their optimal cutoff at presentation were free of death (0%) during follow–up, whereas 53% of those with elevations of all 3 biomarkers had died. For each elevated marker (from 0–3), adjusted analysis suggested a tripling of the risk of death (HR = 2.64; 95%CI, 1.63–4.28; P < .001). Integrated discrimination analyses indicated that the use of these 3 markers in a multimarker approach uniquely improved prediction of death.
Risk for death after admission for acute decompensated heart failure as a function of elevated soluble ST2 (> 35 ng/mL) and N-terminal pro-B-type natriuretic peptide (> 1000 pg/mL) during 4 years of follow-up in the PRIDE study. NT–proBNP, N-terminal pro-B-type natriuretic peptide; sSt2, soluble ST2.
While the baseline sST2 concentration in ADHF tells much about risk for hazard in ADHF, much as serial measurement of BNP or NT–proBNP provides incremental prognostic information in ADHF, emerging data suggest monitoring of sST2 concentrations during in–hospital treatment may afford even greater information regarding risk. Boisot et al44 first reported the value of serial sST2 sampling in a hospitalized patient cohort. In this analysis, the percent change in sST2 level predicted 90–day mortality; notably, those whose ST2 values decreased by 15.5% over the course of hospitalization had 7% mortality compared with 33% mortality among those patients whose ST2 values failed to decrease by 15.5%. Subsequently, in a small trial of hospitalized advanced–stage ADHF patients, any sST2 value above 104 ng/mL measured during the first 48hours of admission to an intensive care unit strongly predicted risk for death, heart transplantation, or mechanical circulatory support (HR = 5.53; P < .001), and was superior to NT–proBNP, galectin-3, or high-sensitivity troponin I (hsTnI).45 In less severely ill patients with ADHF, Manzano–Fernández et al46 reported that both sST2 at presentation and at day 4 of hospitalization were independent predictors of mortality, such that those with sST2 values at baseline and/or day 4 below 76 ng/mL (baseline) or 46 ng/mL (day 4) had lower risks for death. Similarly, Breidthardt et al47 reported that a dynamic change in sST2 value from admission to discharge was a stronger predictor of mortality than baseline values alone in 207 patients with ADHF. These findings all point towards the value of serial measurement of sST2, rather than relying on baseline assessment only.
Curiously, in the Breidthardt et al study,47 treatment with beta–adrenergic blockers appeared to attenuate the risk for mortality predicted by an elevated sST2. These findings are reminiscent of those described previously by Gaggin et al,48 who described the heightened value of beta–adrenergic blockers in those with elevated sST2 concentrations in chronic ambulatory HF. Recently, data have suggested that elevated sST2 may also predict specific benefit from mineralocorticoid receptor antagonist therapy in those recently discharged for ADHF.49 Taken together, these data suggest sST2 monitoring may not only predict risk but also may assist in therapeutic decision–making. This remains to be proven.
Unlike the natriuretic peptides, sST2 concentrations are not affected by age, body mass index, or renal dysfunction, which is a considerable advantage over BNP and NT–proBNP.50 Measurement of sST2 is currently limited to a central laboratory–based enzyme–linked immunosorbent assay that has received regulatory approval in Europe, Asia, and the United States. It is highly likely sST2 will play an important role in the future biomarker–based evaluation and management of ADHF.
Galectin–3Galectin–3 is a soluble peptide secreted by activated macrophages, which activates fibroblasts to secrete collagen, and is an important upstream mediator of fibrosis, including cardiac fibrosis.51 In a rat model, infusion of galectin–3 into the pericardium led to cardiac remodeling and risk for death.52 Staining for galectin–3 in cardiac autopsies or biopsies routinely shows the presence of the protein in the interstitium of remodeled ventricles from patients with HF. Measurement of galectin–3 concentrations is possible in the circulation of patients with HF and may provide important prognostic information; in general, galectin–3 is more prognostic when measured in ADHF than in chronic HF syndromes.
The first study of galectin–3 testing in ADHF was from the PRIDE study.53 In this analysis of 559 patients, higher galectin–3 concentration was a strong independent predictor of 60–day mortality and recurrent HF admission. Subsequent analysis by Shah et al54 of 115 patients with acute dyspnea found that galectin–3 above a median value of 15.0 was a significant predictor of 4–year mortality independent of echocardiographic markers of disease severity and additive to the prognostic power of NT–proBNP. Similarly, De Berardinis et al55 showed galectin–3 to be prognostic in ADHF, complementary to estimation of filling pressures using bio–impedance vector analysis. Galectin–3 may be equally (or incrementally) useful for risk stratification of HFpEF compared with HFrEF56; for example, in 1 study of patients with HFpEF, galectin–3 concentrations above 13.8 ng/mL were a significant predictor of risk after adjustment for age, estimated glomerular filtration rate (eGFR), anemia, diabetes, serum sodium, BNP levels, New York Heart Association functional class, and blood urea nitrogen, respectively (HR = 1.43; 95%CI, 1.07-1.91; P = .015).
Despite these optimistic results, galectin–3 is less prognostic in more chronic HF states, and some studies question its role even in ADHF.57 The results of the galectin–3 assay must be interpreted with caution in the presence of human antimouse antibodies and rheumatoid factor, as well as in patients previously treated with murine monoclonal antibodies, those with known autoimmune disorders, and those with hyperglobulinemia (such as in multiple myeloma), as all of these states can interfere with the assay.58 Additionally, renal dysfunction powerfully undermines the prognostic accuracy of galectin–3 to the point of raising the question of whether the major driver of concentrations of this biomarker is, in fact, renal fibrosis rather than cardiac fibrosis.59 At this point, there is insufficient evidence to support the routine use of galectin–3 in ADHF, largely due to a paucity of adequately–powered studies and contradictory cohort studies.60 Further investigation is necessary to determine the prognostic value of galectin–3 in ADHF.
Highly–sensitive TroponinWhen a patient presents with ADHF, measurement of hsTn is recommended by clinical practice guidelines as well as consensus statements61; this is mainly to seek the presence of type 1 myocardial infarction as the precipitant of ADHF. It is well–established that multiple mechanisms explain elevated hsTn concentrations in patients with ADHF, including acute myocardial infarction; however, a rise and/or fall of hsTn in this setting does not guarantee a type 1 infarction. Nonetheless, knowledge of elevated hsTn in the context of ADHF provides important prognostic information, as above, and may ultimately play an integral role in the multimarker approach to evaluation of HF syndromes, regardless of mechanism.
Compared with conventional troponin assays, hsTn methods detect substantially more myocardial necrosis in patients with HF and are incrementally prognostic, typically providing information that is additive to the natriuretic peptides and other biomarkers such as sST2.
Beyond the already–mentioned study supporting hsTnT in the prognostic evaluation of ADHF43 (Figure 4), other studies show the prognostic utility of this biomarker in ADHF. In a study of 202 patients with ADHF without acute myocardial infarction, hsTnT was detected in 98% of participants (compared with 56% as measured with a conventional TnT method); regardless of conventional TnT, a concentration of hsTnT above 20 pg/mL identified a significantly higher risk of death (HR = 4.7; 95%CI, 1.6-13.8; P = .005). Importantly, hsTnT provides valuable incremental prognostic information when the results of conventional assay are normal.62
Among patients with acute decompensated heart failure, a multimarker strategy including N-terminal pro-B-type natriuretic peptide, soluble ST2, and high-sensitivity troponin T provided independent prognostication, each reclassifying risk. hsTnT: high-sensitivity troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; sST2, soluble ST2. Reproduced with permission from Pascual-Figal et al.43.
For example, in one study, hsTnT was particularly useful among those whose conventional TnT was below 0.03 ng/mL (the lowest cutpoint with < 10% imprecision). Similarly, in those with conventional TnT below the conventional upper reference limit, Parissis et al63 showed that hsTnT provided incremental prognostic information.
With respect to monitoring, serially measured hsTn concentrations64 may inform treatment response and risk for future adverse events, compared with baseline measurements alone. In an analysis performed among 100 patients with ADHF, hsTnT decreased from day 1 to day 3 (P = .04) overall, but this reduction was driven by improvements in concentrations of the biomarker in the group of patients achieving recompensation; in the subgroup of patients who remained decompensated, no significant differences were observed in hsTnT from day 1 to day 3 (P = .96). Similarly, deleterious trends in hsTnI from baseline to discharge may identify those at higher risk for future events.65 In this analysis of a precommercial hsTnI method, 144 patients with ADHF were followed up from admission to 90 days postdischarge. A discharge hsTnI of 23.25 ng/L or higher and BNP of 360 pg/mL or higher were both associated with increased risk for mortality and readmission (P = .003). Patients with increasing hsTnI during treatment had increased mortality compared with patients with stable or decreasing hsTnI (P = .047). Finally, a favorable change in hsTnT concentrations may predict response to therapy with certain drugs for ADHF, such as serelaxin.66
Midregional ProadrenomedullinInitially isolated from human pheochromocytoma cells in the adrenal medulla, adrenomedullin (ADM) is a peptide hormone with natriuretic, vasodilatory, and hypotensive properties that is expressed in many tissue organ systems, including cardiovascular, renal, pulmonary, cerebrovascular, gastrointestinal, and endocrine tissues.67 Serum ADM levels are increased in HF68 and correlate with worsening systolic function, thus increasing pulmonary artery pressures and diastolic dysfunction.69 Plasma concentrations of ADM are also increased in hypertension and chronic renal disease. Infusion of ADM into patients with and without HF resulted in lower mean arterial pressure, increased heart rate, increased cardiac index, and decreased pulmonary capillary wedge pressure. In HF patients in particular, ADM infusion also decreased plasma aldosterone levels. Overall, these results suggest a beneficial, likely compensatory mechanism of ADM in patients with HF.70
An assay to detect the midregional proadrenomedullin (MR-proADM) has been evaluated; MR–proADM is a stable and surrogate measure for mature ADM, and provides useful prognostic information, particularly in the short–term. For example, in the BACH study of patients presenting with acute dyspnea, MR–proADM was superior to both BNP and NT–proBNP in predicting 14–day mortality and improved risk prediction for 90–day mortality when used in conjunction with the natriuretic peptides.71 In the PRIDE study29, MR–proADM was independently prognostic for death, reclassifiying risk at 1 year (HR = 2.70; P < .001) and at 4 years (HR = 1.51; P = .03). In a multinational analysis, MR–proADM added considerable prognostic information beyond a clinical model for 30–day risk; however, its value at 1 year was less robust relative to other markers, such as the natriuretic peptides or sST2 (Figure 1).32 While few data exist on changes in MR–proADM after treatment for ADHF, preliminary data suggest a significant reduction following adequate HF treatment.72 Additional studies are needed to determine the role of MR–proADM in guiding HF treatment.
ProcalcitoninRespiratory disorders may be confused with ADHF, and vice versa. Incorrect treatment for HF in a patient with pneumonia is potentially associated with higher risk, while a missed diagnosis of infection is also similarly grave. Finally, respiratory ailments are a common cause of HF decompensation, and the 2 diagnoses may coexist; in this setting, prognosis may be worse. While natriuretic peptides assist in diagnostic identification of ADHF, the availability of a biomarker to assist in correct diagnosis of severe infection in those with dyspnea would be welcome. Procalcitonin (PCT) is a protein that is upregulated in inflammatory states, especially related to bacterial pathogens73; PCT may therefore be useful in assisting in the diagnostic evaluation of respiratory infection.
In an analysis from the BACH study, Maisel et al74 showed that a diagnostic model using PCT had an AUC for diagnosis of pneumonia of 0.723; as with any biomarker, PCT is not a stand–alone test, and combining physician estimates of the probability of pneumonia with PCT values increased the accuracy to more than 86%. Notably, study participants with ADHF and PCT > 0.21 ng/mL had a worse outcome if not treated with antibiotics (P = .05), while patients with PCT concentrations < 0.05 ng/mL had a better outcome if they did not receive antibiotic therapy (P = .05), supporting the concept of antibiotic stewardship with PCT.74 A subsequent study by investigators in Switzerland found that the use of PCT vs standard care to exclude bacterial infection in HF patients presenting to the emergency department resulted in decreased death and intensive care unit admissions, 4% vs 20% (absolute difference, –16.0%; 95%CI, –28.4% to –3.6%; P = .01) and decreased antibiotic exposure (mean, 3.7 ± 4.0 vs 6.5 ± 4.4; difference, –2.8; 95%CI, –4.4 to –1.2]; P < .01) at 30 days.75
In the BACH analysis, patients with both ADHF and adjudicated pneumonia had the highest PCT values; notably, in a large analysis of 4698 patients from China, those with simple HF had significantly higher PCT levels than healthy controls (0.13μg/L vs 0.04μg/L; P<.01), whereas patients with bacterial infection complicated by ADHF had significantly higher PCT levels than those with simple infection (0.45μg/L vs 0.28μg/L; P<.01). Thus, the results of PCT testing must be interpreted in the context of the severity of a patient's HF.76 These results highlight the need for additional studies.
Renal BiomarkersRenal function is an important prognostic variable in patients with ADHF. Whether abnormal at baseline or worsening after development of ADHF, derangement in measures of renal function, such as blood urea nitrogen, serum creatinine, or eGFR are all potently associated with adverse outcome in ADHF.77,78
More recently, newer biomarkers for estimating renal function such as cystatin C or BTP (protein traza beta), have been examined for their ability to predict mortality in ADHF.79 In a study of 220 participants with ADHF, 53% either died or were rehospitalized for ADHF during follow–up. Those with adverse outcomes had higher BTP (1.04mg/L [range, 0.80–1.49mg/L] vs 0.88mg/L [range, 0.68–1.17mg/L]; P = .003) and cystatin C (1.29mg/L [range, 1.00–1.71mg/L] vs 1.03mg/L [range, 0.86–1.43mg/L]; P = .001). Both BTP (HR = 1.41, 95%CI: 1.06–1.88; P = .018) and cystatin C (HR = 1.50, 95%CI: 1.13–2.01; P = .006) were significant predictors of outcome, whereas serum creatinine, eGFR rate, and blood urea nitrogen were no longer significant.
Both cystatin C and BTP have an advantage over serum creatinine in that they are more sensitive to milder forms of renal dysfunction and, as such, provide more refined risk assessment; indeed, in this analysis, in those with eGFR > 60mL/min/1.73 m2, elevated concentrations of BTP and cystatin C were both associated with a significantly higher risk of adverse clinical events (P < .05). Not surprisingly, researchers recently reported that a cystatin C–based CKD-EPI80 equation to eGFR was superior to an equation based on serum creatinine to prognosticate in ADHF.
Beyond estimating renal function, a number of biomarkers have recently been suggested to be useful to identify renal injury. In this regard, neutrophil gelatinase–associated lipocalin (NGAL), N-acetyl-beta-D-glucosaminidase, and kidney injury molecule-1 have each been examined in ADHF. Besides being of use to prognosticate in this setting, knowledge of risk for acute kidney injury might be useful to avoid nephrotoxic exposures, such as intravenous contrast or certain nephrotoxic drugs.
Maisel et al81 studied 186 patients with ADHF, measuring NGAL at admission; study participants with events had higher levels of NGAL than those without (134 ng/mL vs 84 ng/mL; P < .001). The AUC of the receiver operating characteristic curve for event prediction was higher for NGAL (0.72) than BNP (0.65), serum creatinine (0.57), or eGFR (0.55). In multivariate analyses, NGAL predicted events (P = .001), while neither serum creatinine nor eGFR were significant.
Beyond representing a risk predictor, NGAL measurement may be of use to predict onset of in–hospital WRF (worsening renal function). The GREAT82 investigators recently reported that elevated NGAL in combination with an elevation in one of the natriuretic peptides (BNP or NT–proBNP) was associated with the development of WRF during hospitalization for ADHF; in logistic regression, the combination of elevated natriuretic peptide and NGAL had OR = 2.79 and OR = 3.11 (both P < .04) for NGAL plus BNP or NT–proBNP respectively. This is unsurprising, as the WRF due to type 1 cardiorenal syndrome is typically associated with more severe congestion; thus, the conjoined measurement of a renal injury marker plus a natriuretic peptide would be expected to be more useful than either alone to prognosticate this dreaded complication.83 In addition to predicting in–hospital WRF, NGAL also predicted in–hospital mortality.84
N-acetyl-beta-D-glucosaminidase and kidney injury molecule-1 are urinary biomarkers. While both have been shown to predict all–cause mortality and the composite of mortality and HF rehospitalization in patients with chronic HF, independent of eGFR,85 recent data from the GREAT86 has called their utility for ADHF evaluation into question. More data are needed on the potential role of these biomarkers in ADHF.
CONCLUSIONSIn addition to the diagnostic and prognostic power of the natriuretic peptides for diagnosis, triage and possibly management of ADHF, several other biomarkers have emerged as potentially useful for the practicing clinician. Even in their nascence, several biomarkers have been shown in studies to have significant predictive abilities in ADHF in patients with both HFrEF and HFpEF; among them, sST2 appears the most promising, as it not only adds considerable prognostic information to the natriuretic peptides but also allows for risk monitoring and may provide important treatment information. High–sensitivity troponin also seems promising, although therapeutic decision–making options are yet to be identified for those with an elevated hsTnT or hsTnI. However, measurement of hsTn should always be performed in a patient with ADHF primarily to identify type 1 myocardial infarction as the cause of HF decompensation. Other biomarkers are either falling in stature (eg, galectin–3) or require more data before their use can be supported (eg, MR–proADM). Beyond cardiac biomarkers, several novel markers may be useful to support clinical judgment in the emergency department setting; for example, PCT appears to be a strong candidate for the correct identification of pneumonia in patients with dyspnea and may be useful for stewardship of antibiotics. Renal function markers and renal injury markers may be useful not only to prognosticate but may also assist in therapeutic decision–making, identifying patients most likely to benefit or be harmed by certain therapeutic interventions such as intravenous contrast exposure. As has been the trajectory with the natriuretic peptides, more studies are needed to determine the role of biomarker–guided therapy, but the potential of biomarkers to continue to improve the care of patients with ADHF is substantial.
FUNDINGDr. Januzzi is supported in part by the Desanctis Clinical Scholar Endowment and the Hutter Family Chair in Medicine.
CONFLICTS OF INTERESTJ.L. Januzzi has received significant grant support from Siemens, Thermo–Fisher, and Singulex; modest consulting income from Roche Diagnostics, DiaDexus, Critical Diagnostics, Sphingotec, and Novartis; and significant income from participation in clinical endpoints adjudication committees for Boeringer Ingelheim, Novartis, and Amgen.