The efficacy and safety of ticagrelor vs prasugrel in patients with acute coronary syndromes (ACS) according to body mass index (BMI) remain unstudied. We assessed the efficacy and safety of ticagrelor vs prasugrel in patients with ACS according to BMI.
MethodsPatients (n=3987) were grouped into 3 categories: normal weight (BMI <25kg/m2; n=1084), overweight (BMI ≥ 25 to <30kg/m2; n=1890), and obesity (BMI ≥ 30kg/m2; n=1013). The primary efficacy endpoint was the 1 year incidence of all-cause death, myocardial infarction, or stroke. The secondary safety endpoint was the 1 year incidence of Bleeding Academic Research Consortium type 3 to 5 bleeding.
ResultsThe primary endpoint occurred in 63 patients assigned to ticagrelor and 39 patients assigned to prasugrel in the normal weight group (11.7% vs 7.5%; HR, 1.62; 95%CI, 1.09-2.42; P=.018), 78 patients assigned to ticagrelor and 58 patients assigned to prasugrel in the overweight group (8.3% vs 6.2%; HR, 1.36; 95%CI, 0.97-1.91; P=.076), and 43 patients assigned to ticagrelor and 37 patients assigned to prasugrel in the obesity group (8.6% vs 7.3%; HR, 1.18; 95%CI, 0.76-1.84; P=.451). The 1-year incidence of bleeding events did not differ between ticagrelor and prasugrel in patients with normal weight (6.5% vs 6.6%; P=.990), overweight (5.6% vs 5.0%; P=.566) or obesity (4.4% vs 2.8%; P=.219). There was no significant treatment arm-by-BMI interaction regarding the primary endpoint (Pint=.578) or secondary endpoint (Pint=.596).
ConclusionsIn patients with ACS, BMI did not significantly impact the treatment effect of ticagrelor vs prasugrel in terms of efficacy or safety.
Clinical Trial Registration: NCT01944800.
Keywords
Obesity is an important risk factor for the development of cardiovascular disease.1 Obese and overweight patients have an increased risk for developing an acute coronary syndrome (ACS)2 and several studies3–7 have shown that most patients undergoing percutaneous coronary intervention (PCI) are overweight or obese. Dual antiplatelet therapy with a P2Y12 inhibitor and aspirin is the cornerstone of treatment for patients with ACS undergoing PCI. Previous studies have suggested that body mass index (BMI) may impact on the response to platelet P2Y12 receptor inhibitors, mostly to clopidogrel, with higher on-treatment platelet reactivity observed in overweight and obese patients.8–14 Overweight patients may need a higher loading dose of clopidogrel to adequately inhibit platelet aggregation15,16 and BMI> 30kg/m2 has been identified as an independent predictor of impaired response to clopidogrel.17 Randomized clinical trials have shown the superiority of prasugrel18 and ticagrelor19 over clopidogrel in terms of reducing the risk of ischemic events in patients with ACS undergoing an invasive treatment. These trials indicated that the clinical efficacy and safety of prasugrel and ticagrelor may differ according to BMI in patients with ACS compared with clopidogrel. However, due to the lack of head-to-head comparative studies, the impact of BMI on the efficacy and safety of ticagrelor vs prasugrel in patients with ACS undergoing invasive treatment remains unknown. The ISAR-REACT (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment) 5 trial compared the efficacy and safety of ticagrelor vs prasugrel in patients presenting with ACS and scheduled to undergo an invasive treatment.20 Herein, we present the results of this post-hoc analysis comparing the efficacy and safety of ticagrelor vs prasugrel according to BMI.
METHODSStudy population and designThis study is a post-hoc analysis of the ISAR-REACT 5 trial and includes all patients with available BMI data. The design and outcomes of the ISAR-REACT 5 trial have previously been reported.20,21 Patients were eligible for enrollment if they were hospitalized for an ACS (ST-segment elevation myocardial infarction [STEMI], non–ST-segment elevation myocardial infarction [NSTEMI], or unstable angina) and were scheduled to undergo invasive evaluation. Patients were randomly assigned to either ticagrelor or prasugrel in a 1:1 ratio. The study had an open-label design. Patients in the ticagrelor arm received a loading dose of 180mg of ticagrelor as soon as possible after randomization and continued at a maintenance dose of 90mg twice daily. Patients in the prasugrel arm received a loading dose of 60mg of prasugrel (immediately after randomization in patients with STEMI, and after coronary angiography was known [ie, after coronary angiography] but before PCI in patients with NSTEMI or unstable angina) and continued at a maintenance dose of 10mg daily. For patients aged ≥ 75 years or those with a body weight lower than 60kg, a reduced maintenance dose of 5mg of prasugrel was recommended.22 All patients received a loading dose of 150 to 300mg of intravenous or chewed aspirin and a maintenance dose of 75 to 100mg once daily. Written informed consent was obtained from all patients. The study protocol was approved by the local ethics committee at each participating center. The study conformed to the Declaration of Helsinki.
Definitions and endpointsBMI was calculated as a ratio of weight in kilograms to height in meters squared (kg/m2) using the patient's weight and height measured during the hospital stay. BMI data were available in 3987 of 4018 patients of the ISAR-REACT 5 trial. BMI ranged from 14.0kg/m2 to 55.0kg/m2. Patients were categorized into groups according to BMI categories: patients with a BMI of <25kg/m2 (normal weight group), patients with a BMI of ≥ 25kg/m2 to <30kg/m2 (overweight group), and patients with a BMI of ≥ 30kg/m2 (obesity group). Of the 1084 patients in the normal weight group, 27 patients (2.5% of the group with BMI <25kg/m2) had a BMI <18.5kg/m2.
The primary (efficacy) endpoint was the 12-month incidence of all-cause death, myocardial infarction, or stroke. The secondary (safety) endpoint was the 12-month incidence of Bleeding Academic Research Consortium (BARC) type 3 to 5 bleeding.23 Myocardial infarction was defined according to the Third Universal Definition of Myocardial Infarction.24 Stent thrombosis and cardiovascular death were defined according to the Academic Research Consortium (ARC) criteria.25 Detailed definitions are provided in the . All outcomes assessed in the primary trial20 were included in this analysis. All primary and secondary outcomes were centrally adjudicated in a blinded manner by members of the event adjudication committee.
Follow-upFollow-up was scheduled at 1 month, 6 months, and 1 year. In case of potential endpoint-related adverse events, source data were solicited. All serious adverse events, including outcomes analyzed in this study, were monitored on-site. Patients were monitored either via hospital visits, outpatient visits or through telephone and structured follow-up letters.
Statistical analysisThis study represents a post-hoc analysis of the ISAR-REACT 5 trial. Continuous variables are presented as mean with standard deviation or median with 25th-75th percentiles and were compared using the Student t test or the Wilcoxon rank sum test as appropriate. Categorical data are presented as counts and proportions and were compared using the chi-squared test. The primary endpoint and all-cause death are presented as cumulative incidence(s) and were calculated using the Kaplan-Meier method.26,27 All other endpoints are presented as cumulative incidence(s) after accounting for the competing risk of death. Competing risk analysis is used when there is an event (in our study death) whose occurrence precludes the occurrence of the primary event of interest.28 The comparison between the groups was performed using the Cox proportional-hazards model with the participating center and stratification according to clinical presentation (ST-segment elevation or non–ST-segment elevation ACS) entered into the model as covariates along with the study drug. The association of study drug and BMI category with the primary and secondary endpoints was adjusted for potential confounders using the Cox proportional-hazards model. The following variables were entered into the model: study drug, age, sex, diabetes mellitus, smoking, hypertension, hypercholesterolemia, history of MI, history of PCI, history of coronary artery bypass grafting, cardiogenic shock, heart rate, serum creatinine on admission, diagnosis on admission, type of treatment (conservative or PCI), prasugrel 5mg and treatment arm-by-BMI interaction. To assess the treatment arm-by-BMI interaction for study endpoints, an interaction term was entered into the Cox proportional-hazards model. Risk estimates are presented as hazard ratios [HR] with 95% confidence intervals [95%CI]. The primary (efficacy) endpoint was analyzed according to the intention-to-treat principle (ie, including all patients as initially assigned, irrespective of the actual treatment received). The secondary (safety) endpoint was analyzed in a modified intention-to-treat (mITT) population (ie, including all patients with at least 1 application of the study drug with bleeding assessed for up to 7 days after study drug discontinuation). The statistical analysis was performed using the R 3.6.0 Statistical Package (The R foundation for Statistical Computing, Vienna, Austria). A 2-sided P <.05 was considered to indicate statistical significance.
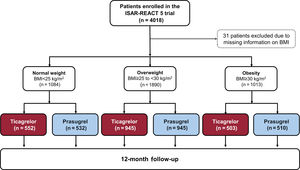
RESULTSBaseline and outcome data according to BMI categoriesThe study flowchart is shown in figure 1. Data on BMI were available in 3987 patients: 1084 patients with normal weight, 1890 patients with overweight and 1013 patients with obesity. Baseline data are shown in . Patients with obesity were younger, less likely to be smokers and more likely to have diabetes, hypertension, hypercholesterolemia, or prior PCI. Cardiogenic shock was less frequent and serum creatinine values were higher in patients with obesity. The rate of coronary angiography and treatment strategy differed little across the BMI categories. Angiographic and procedural data are shown in and . Drug therapy at discharge is shown in . Twenty-two of the 27 patients with a BMI <18.5kg/m2 were discharged on ticagrelor and 5 patients were discharged on prasugrel. Of the latter, 4 patients were discharged on a reduced dose of prasugrel (5mg). In patients with normal weight, overweight, and obesity, the primary endpoint occurred in 102 (9.6%), 136 (7.3%), and 80 (8.0%) patients, respectively (hazard ratio [HR]=0.77; 95%CI, 0.59-0.99; P=.047 for overweight vs normal weight and HR, 0.84; 95%CI, 0.62-1.13; P=.240 for obesity vs normal weight; ). The secondary endpoint (BARC type 3-5 bleeding) occurred in 79 (7.4%), 104 (5.6%) and 41 (4.1%) patients, respectively (HR, 0.72; 95%CI, 0.54-0.97; P=.031 for overweight vs normal weight and HR, 0.52; 95%CI, 0.36-0.77; P=.0008 for obesity vs normal weight; .
Baseline data according to study drugs and BMI categoriesBaseline data in patients assigned to ticagrelor or prasugrel in each BMI category are shown in table 1. In the group with normal weight, the proportion of patients with hypertension and prior PCI was significantly higher among ticagrelor-assigned patients. In the overweight group, baseline characteristics were well-balanced between the 2 treatment arms. In the group with obesity, patients assigned to ticagrelor were younger than those assigned to prasugrel. The remaining characteristics did differ little between patients assigned to ticagrelor and prasugrel across all BMI categories.
Baseline characteristics
| Characteristic | Normal weight(BMI <25 kg/m2)(n=1084) | Overweight(BMI ≥ 25 to <30 kg/m2)(n=1890) | Obesity(BMI ≥ 30 kg/m2)(n=1013) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ticagrelor(n=552) | Prasugrel(n=532) | P | Ticagrelor(n=945) | Prasugrel(n=945) | P | Ticagrelor(n=503) | Prasugrel(n=510) | P | |
| Age, y | 67.4±11.8 | 66.4±12.2 | .165 | 64.5±11.8 | 64.5±11.7 | .961 | 61.3±11.9 | 62.8±12.2 | .043 |
| Sex | .504 | .313 | .787 | ||||||
| Female | 189 (34.2) | 171 (32.1) | 165 (17.5) | 183 (19.4) | 122 (24.3) | 119 (23.3) | |||
| Male | 363 (65.8) | 361 (67.9) | 780 (82.5) | 762 (80.6) | 381 (75.7) | 391 (76.7) | |||
| Diabetes | 88 (15.9) | 74/531 (13.9) | .401 | 189 (20.0) | 189 (20.0) | > .999 | 182/502 (36.3) | 160 (31.4) | .115 |
| Insulin-treated | 25 (4.5) | 24/531 (4.5) | > .999 | 60 (6.4) | 55 (5.8) | .700 | 58/502(11.6) | 56 (11.0) | .850 |
| Smoker | 190/548 (34.7) | 207/529 (39.1) | .146 | 325/942 (34.5) | 297/942 (31.5) | .186 | 163/500 (32.6) | 157/509 (30.8) | .595 |
| Hypertension | 375/551 (68.1) | 324/531 (61.0) | .018 | 654/944 (69.3) | 638/943 (67.7) | .478 | 397/501 (79.2) | 407 (79.8) | .886 |
| Hypercholesterolemia | 314/550(57.1) | 277/530 (52.3) | .126 | 528/944 (55.9) | 541/944 (57.3) | .577 | 326/501 (65.1) | 334 (65.5) | .941 |
| Prior myocardial infarction | 85/551(15.4) | 72/532(13.5) | .425 | 146 /944 (15.5) | 153/944 (16.2) | .705 | 79 (15.7) | 92 (18.0) | .364 |
| Prior PCI | 117/551 (21.2) | 84/531(15.8) | .027 | 213 (22.5) | 234/944 (24.8) | .273 | 122 (24.3) | 141 (27.6) | .246 |
| Prior CABG | 28/551 (5.1) | 26/531 (4.9) | > .999 | 53 (5.6) | 66 (7.0) | .256 | 33 (6.6) | 34 (6.7) | > .999 |
| Cardiogenic shock | 10 (1.8) | 16 (3.0) | .277 | 16 (1.7) | 14 (1.5) | .854 | 5 (1.0) | 3 (0.6) | .503 |
| Systolic blood pressure, mmHg | 143±24.4 | 142±24.5 | .498 | 143±24.8 | 142±24.0 | .473 | 146±25.8 | 145±25.1 | .698 |
| Diastolic blood pressure, mmHg | 80.7±14.6 | 81.2±13.5 | .555 | 81.8±14.1 | 81.9±13.9 | .845 | 83.8±15.3 | 82.1±14.2 | .072 |
| Heart rate, beats/min | 77.1±16.4 | 76.8±16.9 | .762 | 76.4±15.9 | 75.0±15.0 | .056 | 78.1±15.4 | 77.1±14.9 | .297 |
| Body mass index, kg/m2 | 23.5[22.0-24.4] | 23.5[22.0-24.4] | .914 | 27.3[26.1-28.4] | 27.3[26.1-28.4] | .479 | 32.8[31.1-35.5] | 32.7[31.1-35.5] | .852 |
| Weight <60 kg | 107 (19.4) | 91 (17.1) | .372 | 1 (0.1) | 3 (0.3) | .625 | 0 | 0 | |
| Creatinine, μmol/L | 83.6±23.6 | 85.0±33.9 | .418 | 89.5±28.4 | 88.1±28.0 | .254 | 88.9±29.0 | 91.6±30.7 | .153 |
| Diagnosis at admission | .547 | .498 | .252 | ||||||
| Unstable angina | 71 (12.9) | 57 (10.7) | 114 (12.1) | 124 (13.1) | 64 (12.7) | 80 (15.7) | |||
| NSTEMI | 245 (44.4) | 243 (45.7) | 437 (46.2) | 451 (47.7) | 242 (48.1) | 223 (43.7) | |||
| STEMI | 236 (42.7) | 232 (43.6) | 394 (41.7) | 370 (39.2) | 197 (39.2) | 207 (40.6) | |||
| Coronary angiography | 548 (99.3) | 531 (99.8) | .374 | 942 (99.7) | 943 (99.8) | > .999 | 501 (99.6) | 508 (99.6) | >.999 |
| Treatment strategy | .475 | .091 | .506 | ||||||
| PCI | 453 (82.2) | 451 (84.8) | 791 (84.0) | 819 (86.7) | 421 (83.7) | 412 (80.9) | |||
| CABG | 10 (1.8) | 10 (1.9) | 26 (2.7) | 14 (1.5) | 11 (2.2) | 12 (2.4) | |||
| Conservative | 88 (16.0) | 71 (13.3) | 125 (13.3) | 112 (11.9) | 71 (14.1) | 85 (16.7) | |||
BMI, body mass index; CABG, coronary artery bypass grafting; NSTEMI, non–ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Data are expressed as No. (%) or mean±standard deviation, median with 25th-75th percentiles or counts (%).
Missing continuous data:
Normal weight group (BMI <25kg/m2): diastolic blood pressure: 1 patient in the ticagrelor group, 3 patients in the prasugrel group.
Overweight group (BMI ≥ 25 to <30kg/m2): systolic blood pressure: 2 patients (1 patient in each group), diastolic blood pressure: 7 patients (5 in the ticagrelor group, 2 patients in the prasugrel group).
Obesity group (BMI≥ 30kg/m2): systolic blood pressure: 1 patient in the prasugrel group, diastolic blood pressure: 3 patients (1 in the ticagrelor group, 2 patients in the prasugrel group). The remaining continuous data were complete.
Angiographic data are shown in . There were no significant differences in terms of access site, number of narrowed coronary arteries, or left ventricular ejection fraction between patients according to study drugs in groups with normal weight, overweight, or obesity. Procedural characteristics are shown in . Drug therapy at discharge is shown in . In the normal weight and overweight groups, patients assigned to prasugrel were more often discharged on aspirin (P=.023) and clopidogrel (P=.049), respectively.
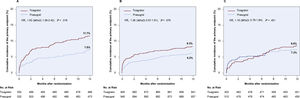
Clinical outcomesClinical outcomes according to study drugs across BMI categories are shown in table 2. In the group with normal weight, the primary endpoint occurred in 63 patients (11.7%) in the ticagrelor group and 39 patients (7.5%) in the prasugrel group (HR, 1.62; 95%CI, 1.09-2.42; P=.018; figure 2A). In the overweight group, the primary endpoint occurred in 78 patients (8.3%) in the ticagrelor group and 58 patients (6.2%) in the prasugrel group (HR, 1.36; 95%CI, 0.97-1.91; P=.076; figure 2B). In the group with obesity, the primary endpoint occurred in 43 patients (8.6%) in the ticagrelor group and 37 patients (7.3%) in the prasugrel group (HR, 1.18; 95%CI, 0.76-1.84; P=.451; figure 2C). There was no treatment arm-by-BMI interaction regarding the primary endpoint (P for interaction=.578).There were no significant differences regarding the incidence of death between ticagrelor and prasugrel across the BMI categories. In the group with normal weight, the incidence of myocardial infarction, and definite or probable stent thrombosis was higher in patients assigned to ticagrelor than those assigned to prasugrel (5.9% vs 2.5%; HR, 2.43; 95%CI, 1.27-4.63; P=.007), and (2.0% vs 0.4%; HR, 5.22; 95%CI, 1.15-23.62; P=.032), respectively. In the overweight group, the incidence of myocardial infarction, and definite or probable stent thrombosis in the ticagrelor and prasugrel groups was 3.3% vs 2.9%; HR, 1.18; 95%CI, 0.71-1.99; P=.520, and 1.1% vs 1.4%; HR, 0.79; 95%CI, 0.34-1.79; P=.567, respectively. In the group with obesity, the incidence of myocardial infarction, and definite or probable stent thrombosis in the ticagrelor and prasugrel groups was 6.6% vs 4.0%; HR, 1.68; 95%CI, 0.96-2.93; P=.067, and 1.0% vs 1.0%; HR, 1.01; 95%CI, 0.29-3.48; P=.991, respectively.
Clinical outcomes
| Outcome | Normal weight(BMI <25 kg/m2)(n=1084) | Overweight(BMI ≥ 25 to <30 kg/m2)(n=1890) | Obesity(BMI ≥ 30 kg/m2)(n=1013) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ticagrelor(n=552) | Prasugrel(n=532) | HR(95%CI) | P | Ticagrelor(n=945) | Prasugrel(n=945) | HR(95%CI) | P | Ticagrelor(n=503) | Prasugrel(n=510) | HR(95%CI) | P | |
| Primary endpoint (death,myocardial infarction,or stroke) | 63 (11.7) | 39 (7.5) | 1.62(1.09-2.42) | .018 | 78 (8.3) | 58 (6.2) | 1.36(0.97-1.91) | .076 | 43 (8.6) | 37 (7.3) | 1.18(0.76-1.84) | .451 |
| Death | 34 (6.3) | 28 (5.3) | 1.22(0.74-2.02) | .429 | 42 (4.5) | 26 (2.8) | 1.59(0.97-2.59) | .065 | 14 (2.8) | 16 (3.2) | 0.89(0.44-1.83) | .760 |
| Cardiovascular | 24 | 23 | 27 | 21 | 12 | 13 | ||||||
| Noncardiovascular | 10 | 5 | 15 | 5 | 2 | 3 | ||||||
| Myocardial Infarction | 32 (5.9) | 13 (2.5) | 2.43(1.27-4.63) | .007 | 31 (3.3) | 27 (2.9) | 1.18(0.71-1.99) | .520 | 33 (6.6) | 20 (4.0) | 1.68(0.96-2.93) | .067 |
| Type 1 | 16 | 7 | 12 | 16 | 24 | 12 | ||||||
| Type 2 | 1 | 2 | 1 | 1 | 2 | 0 | ||||||
| Type 4a | 6 | 2 | 10 | 4 | 3 | 5 | ||||||
| Type 4b | 8 | 2 | 8 | 6 | 4 | 3 | ||||||
| Type 5 | 1 | 0 | 0 | 0 | 0 | 0 | ||||||
| STEMI | 13 | 3 | 10 | 5 | 8 | 6 | ||||||
| Stroke | 8 (1.5) | 4 (0.8) | 1.92(0.58-6.39) | .287 | 13(1.4) | 10 (1.1) | 1.32(0.58-3.01) | .514 | 1 (0.2) | 5 (1.0) | 0.21(0.02-1.77) | .151 |
| Ischemic | 6 | 3 | 9 | 10 | 1 | 4 | ||||||
| Hemorrhagic | 2 | 1 | 4 | 0 | 0 | 1 | ||||||
| Definite or probable stent thrombosis | 11 (2.0) | 2 (0.4) | 5.22(1.15-23.62) | .032 | 10 (1.1) | 13 (1.4) | 0.79(0.34-1.79) | .567 | 5 (1.0) | 5 (1.0) | 1.01(0.29-3.48) | .991 |
| Definite stent thrombosis | 9 (1.7) | 2 (0.4) | 4.17(0.89-19.37) | .069 | 8 (0.8) | 7 (0.7) | 1.18(0.43-3.27) | .746 | 5 (1.0) | 3 (0.6) | 1.71(0.41-7.16) | .464 |
| BARC type 3 to 5 bleeding* | 30/541 (6.5) | 29/472(6.6) | 1.00(0.60-1.67) | .990 | 45/935 (5.6) | 39/849 (5.0) | 1.13(0.74-1.74) | .566 | 19/501(4.4) | 11/434 (2.8) | 1.59(0.76-3.35) | .219 |
| 3a | 14 | 10 | 21 | 25 | 12 | 6 | ||||||
| 3b | 11 | 16 | 14 | 9 | 6 | 5 | ||||||
| 3c | 1 | 0 | 3 | 2 | 0 | 0 | ||||||
| 4 | 3 | 0 | 4 | 2 | 1 | 0 | ||||||
| 5a | 0 | 0 | 1 | 0 | 0 | 0 | ||||||
| 5b | 1 | 3 | 2 | 1 | 0 | 0 | ||||||
95%CI, confidence interval; BARC, Bleeding Academic Research Consortium; BMI, body mass index; HR, hazard ratio; STEMI, ST-segment elevation myocardial infarction.
Data are numbers of events with Kaplan-Meier estimates (%) for the primary endpoint and death or cumulative incidence (%) after accounting for the competing risk of death for the remaining endpoints.
Cumulative incidence of the primary endpoint (1-year incidence of death, myocardial infarction, or stroke) in the ticagrelor and prasugrel groups in A) patients with normal weight, B) patients with overweight and C) patients with obesity. 95%CI, 95% confidence interval; HR, hazard ratio.
Bleeding events are shown in table 2. In the group with normal weight, BARC type 3 to 5 bleeding occurred in 30 patients assigned to ticagrelor and 29 patients assigned to prasugrel (cumulative incidence accounting for competing risk 6.5% and 6.6%, respectively; HR, 1.00; 95%CI, 0.60-1.67; P=.990). In the overweight group, BARC type 3 to 5 bleeding occurred in 45 patients assigned to ticagrelor and 39 patients assigned to prasugrel (cumulative incidence accounting for competing risk, 5.6% and 5.0%, respectively; HR, 1.13; 95%CI, 0.74-1.74; P=.566). In the group with obesity, BARC type 3 to 5 bleeding occurred in 19 patients in the ticagrelor group and 11 patients in the prasugrel group (cumulative incidence accounting for competing risk, 4.4% and 2.8%, respectively; HR, 1.59 95%CI, 0.76-3.35; P=.219). Time-to-event curves are shown in figure 3. There was no treatment arm-by-BMI interaction regarding the secondary endpoint (P for interaction=.596).
Cumulative incidence of the secondary endpoint (1-year incidence of Bleeding Academic Research Consortium type 3-5 bleeding) accounting for the competing risk of death in the ticagrelor and prasugrel groups in A) patients with normal weight, B) patients with overweight and C) patients with obesity. BARC, Bleeding Academic Research Consortium; 95%CI, 95% confidence interval; HR, hazard ratio.
To address an eventual impact of underweight patients (those with BMI <18.5kg/m2; n=27 patients; 2.5% of the group with BMI <25kg/m2), a sensitivity analysis was performed by excluding these patients from the group with normal weight (BMI <25kg/m2). Among patients with a BMI between 18.5kg/m2 and 25kg/m2 (n=1057 patients), the primary and secondary endpoints occurred in 99 patients (9.5%) and 76 patients (7.3%), respectively. The primary endpoint occurred in 60/532 patients assigned to ticagrelor and 39/525 patients assigned to prasugrel (11.5% vs 7.6%; HR, 1.58; 95%CI, 1.06-2.37; P=.026). The secondary endpoint (BARC type 3 to 5 bleeding) occurred in 28/521 patients assigned to ticagrelor and 29/466 patients assigned to prasugrel (6.5% vs 6.6%; HR, 0.96; 95%CI, 0.57-1.61; P=.877). Thus, in the current analysis, the exclusion of patients with a BMI <18.5kg/m2 appears to have no impact on the frequency of adverse events or on the efficacy and safety of ticagrelor vs prasugrel among patients with a BMI <25kg/m2.
The association of study drug and BMI category with the primary and secondary outcomes was adjusted for potential confounders using the multivariable Cox proportional-hazards model (see methods for variables entered in the model). After adjustment for potential confounders, ticagrelor was independently associated with the primary (adjusted HR, 1.52, 95%CI, 1.13-2.04), but not with the secondary (adjusted HR, 1.01; 95%CI, 0.71-1.37) endpoint. BMI category was not associated with the primary endpoint (adjusted HR, 0.88; 95%CI, 0.67-1.14; P=.331 for overweight vs normal weight and HR=1.07 [0.78-1.46]; P=.696 for obesity vs normal weight); however, it appears to be associated with the secondary endpoint (HR, 0.87; 95%CI, 0.64-1.18; P=.365 for overweight vs normal weight and HR, 0.64; 95%CI, 0.43-0.96; P=.031 for obesity vs normal weight).
DISCUSSIONThe main findings of this study can be summarized as follows: a) In patients with ACS, BMI did not significantly impact the treatment effect of ticagrelor vs prasugrel in terms of efficacy or safety. The benefit of prasugrel over ticagrelor in reducing the 1-year incidence of the composite endpoint of death, myocardial infarction, or stroke was directionally consistent across all BMI categories, albeit with different risk estimates. Differences in the primary outcome between ticagrelor and prasugrel were mostly driven by differences in the occurrence of myocardial infarction. b) The risk of bleeding appears to be similar between ticagrelor and prasugrel across all BMI categories, although this post-hoc analysis might be underpowered to evaluate differences in terms of safety.
The impact of BMI on the efficacy and safety of ticagrelor vs prasugrel in patients with ACS so far has not been investigated. Thus, the main focus of the current study was to compare the efficacy and safety of ticagrelor vs prasugrel according to BMI categories in these patients. Randomized trials provided some evidence that the efficacy and safety of prasugrel and ticagrelor may differ according to body weight or BMI in patients with ACS undergoing PCI. In the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel—Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38, no net clinical benefit of prasugrel compared with clopidogrel was observed in patients with a body weight of <60kg.18 As a result, a reduced maintenance dose of 5mg of prasugrel in patients with a body weight of <60kg was recommended.18 In the PLATelet inhibition and patient Outcomes (PLATO) trial, the benefit of ticagrelor in terms of reduction of ischemic events was attenuated in patients weighing less than the median weight for either sex (P for interaction=.04), whereas in patients weighing ≥ 80kg, the benefit of ticagrelor vs clopidogrel was consistent with the overall trial results.19 However, in the PLATO trial, the risk of bleeding was higher with ticagrelor than with clopidogrel in patients with a BMI ≥ 30kg/m2.19 Due to the lack of a direct comparison, differences in the baseline characteristics of patients treated with prasugrel or ticagrelor and different metrics (body weight or BMI) investigated in the 2 trials, the question of whether prasugrel and ticagrelor differ in their efficacy and safety according to BMI in patients with ACS undergoing PCI remains unanswered.
Our study found no significant treatment arm-by-BMI interaction regarding the primary endpoint and the benefit of prasugrel over ticagrelor in reducing the primary endpoint was consistent with the overall trial results. However, the benefit of prasugrel appears to be somewhat more evident in patients with normal weight. Although the exact reasons for this finding remain unclear, some studies have suggested that response to prasugrel and ticagrelor may differ according to body size indices or BMI. A pharmacodynamic study by Jakubowski et al.12 showed a consistent inverse relationship between body weight and response to clopidogrel and prasugrel, suggesting that in patients with lower body weight the response to treatment with prasugrel or clopidogrel is higher. Other studies have shown that BMI strongly impacts the response to clopidogrel and prasugrel, with a higher incidence of high on-treatment platelet reactivity in obese patients after coronary stenting.9,10,29 These studies strongly suggested that in patients with low body weight the response to prasugrel is preserved or even enhanced.
Data on the impact of BMI on the response to ticagrelor are limited and controversial. A prior meta-analysis by Alexopoulos et al.13 showed higher platelet reactivity with increasing BMI in patients on a ticagrelor maintenance dose. Conversely, Deharo et al.9 suggested that BMI does not influence the extent of platelet inhibition by ticagrelor. A recent subgroup analysis of the GLOBAL LEADERS trial showed that, overall, BMI did not influence the treatment effect of ticagrelor monotherapy.30 However, in patients with ACS, beneficial effects of ticagrelor monotherapy in terms of reduction of the primary endpoint (2-year incidence of death or new Q-wave myocardial infarction) were seen in patients with a BMI of <27kg/m2 (Pint=.047).30 Contrary to these findings, the PLATO trial showed an attenuation of efficacy of ticagrelor compared with clopidogrel in patients with ACS and a body weight under the median value.19 Finally, given a previous study that showed comparable outcomes in diabetic patients assigned to ticagrelor or prasugrel,31 perhaps the higher proportion of patients with diabetes in the overweight/obesity groups may have contributed to the findings observed in the current analysis. In all, although there are some explanations why the benefit of prasugrel over ticagrelor in reducing the ischemic risk may be somewhat more evident in the normal weight group, but less evident in the overweight and obesity groups, a clear-cut explanation for these findings is missing and further studies are needed to tease out the underlying mechanisms. In addition to reduction of overall ischemic events, the significant reduction in the incidence of myocardial infarction and probable or definite stent thrombosis by prasugrel in patients with normal weight may further suggest that prasugrel offers stronger anti-ischemic/antithrombotic protection in patients with ACS after PCI in this BMI category compared with ticagrelor.
The present study shows that ticagrelor and prasugrel were associated with a similar bleeding risk across all BMI categories. Evidence on the safety profile (bleeding risk) of ticagrelor vs prasugrel in different BMI categories in patients with ACS after PCI remains limited, indirect (mostly obtained from comparison with clopidogrel for either drug) and controversial.18,19 In the TRITON-TIMI 38 trial, patients weighing less than 60kg had no net benefit from prasugrel, owing to the high risk of major bleeding in this group.18 Conversely, the PLATO trial reported a significant 21% higher risk of bleeding with ticagrelor compared with clopidogrel in patients with BMI ≥ 30kg/m2 compared with patients with BMI <30kg/m2 (Pint=.05).19 Our study, which performed a direct comparison between ticagrelor and prasugrel, did not find any significant differences between ticagrelor and prasugrel in terms of bleeding risk across all BMI categories. In this regard, our data are reassuring and important, considering that bleeding has recently received considerable attention and has been defined as a primary endpoint in trials of de-escalation strategies of antiplatelet drugs.
LimitationsOur study has limitations. The present analysis is a post-hoc analysis of a randomized trial. As such, it carries the inherent limitations of post-hoc analyses. The categorization of patients in groups according to BMI reduced the study power to detect differences in the efficacy and safety of ticagrelor vs prasugrel in BMI categories. In the normal weight group, 2.5% of patients had a BMI <18.5kg/m2. The sensitivity analysis showed that the exclusion of patients with a BMI <18.5kg/m2 from the group with normal weight had almost no impact (0.1% difference) on the occurrence of primary or secondary endpoints or on the efficacy or safety of ticagrelor vs prasugrel. However, these data do not negate an impact of underweight on the occurrence of adverse events or on the efficacy and safety of ticagrelor vs prasugrel in patients with ACS; they simply show that the proportion of underweight patients was too small to have an overall impact on the occurrence of events in the current study. Furthermore, the observed greater reduction of ischemic events by prasugrel as compared with ticagrelor in patients with normal weight may be the result of chance amplified by multiple testing.32 Since randomization was not performed according to BMI, a possible impact of hidden confounders cannot be entirely ruled out even though we adjusted for an array of clinical and demographic variables in the multivariable analysis. Although the treatment effect of ticagrelor vs prasugrel was directionally consistent, the study was underpowered to assess heterogeneity in the treatment effect across BMI categories. Finally, although all adverse events were centrally adjudicated in a blinded manner, the study had an open-label design. For these reasons, the study findings should be considered as exploratory or hypothesis-generating.
ConclusionsIn conclusion, in patients with ACS, BMI did not impact significantly on the treatment effect of ticagrelor vs prasugrel in terms of efficacy or safety. The benefit of prasugrel over ticagrelor in reducing the 1-year incidence of the composite endpoint of death, myocardial infarction, or stroke was directionally consistent across all BMI categories, albeit with different risk estimates. The risk of bleeding appears to be similar between ticagrelor and prasugrel across all BMI categories, although our analysis might be underpowered to evaluate differences in terms of safety.
- –
Overweight and obese patients presenting with an ACS have an increased risk of ischemic events compared with normal weight patients.
- –
BMI impacts on the response to platelet P2Y12 receptor inhibitors.
- –
Overweight and obese patients are often poor responders to antiplatelet drugs (mostly to clopidogrel) and may benefit from more potent platelet inhibition strategies.
- –
The efficacy and safety of ticagrelor vs prasugrel in patients with ACS according to BMI are unknown.
- –
In patients with ACS, BMI did not impact significantly on the treatment effect of ticagrelor vs prasugrel in terms of efficacy or safety.
- –
The superiority of prasugrel over ticagrelor in reducing the 1-year incidence of the composite endpoint of death, myocardial infarction, or stroke was directionally consistent across all BMI categories, albeit with different risk estimates.
- –
The bleeding risk was similar between ticagrelor and prasugrel across all BMI categories.
- –
BMI may not be used to guide selection between prasugrel and ticagrelor for patients with ACS, managed with an invasive strategy.
This work was supported by a grant (FKZ 81X1600501) from the German Center for Cardiovascular Research and the Deutsches Herzzentrum München, Germany. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report
AUTHORS’ CONTRIBUTIONSConceptualization, methodology, funding: A. Kastrati, S. Schüpke. Methodology: G. Ndrepepa, D.J. Angiolillo, S. Schüpke, A. Kastrati. Validation: S. Lahu, G. Ndrepepa, K. Mayer. Formal analysis: A. Kastrati. Resources: F.-J. Neumann, D. Sibbing, I. Bernlochner, M. Menichelli, G. Richardt, S. Gewalt, J.J. Coughlan, A. Aytekin, B. Witzenbichler, W. Hochholzer, S. Cassese, S. Kufner, E. Xhepa, H.B. Sager, M. Joner, M. Fusaro, K.-L. Laugwitz, H. Schunkert, A. Akin. Writing-original draft: S. Lahu. Writing-review & editing: M. Behnes, G. Ndrepepa, F.-J. Neumann, D. Sibbing, I. Bernlochner, M. Menichelli, K. Mayer, G. Richardt, S. Gewalt, D.J. Angiolillo, J.J. Coughlan, A. Aytekin, B. Witzenbichler, W. Hochholzer, S. Cassese, S. Kufner, E. Xhepa, H.B. Sager, M. Joner, M. Fusaro, K.-L. Laugwitz, H. Schunkert, S. Schüpke, A. Kastrati, A. Akin. Visualization: S. Lahu, G. Ndrepepa. Supervision: F.-J. Neumann, D. Sibbing, I. Bernlochner, M. Menichelli, K. Mayer, G. Richardt, B. Witzenbichler, K.-L. Laugwitz, H. Schunkert, S. Schüpke, A. Kastrati, A. Akin. Project administration: M. Behnes, F.-J. Neumann, D. Sibbing, I. Bernlochner, M. Menichelli, K. Mayer, G. Richardt, B. Witzenbichler, K.-L. Laugwitz, H. Schunkert, S. Schüpke, A. Kastrati, A. Akin. Final approval of the version to be published: all authors. Agreement to be accountable for all aspects of the work: all authors. All of the authors meet the criteria for authorship defined by International Committee of Medical Journal Editors.
CONFLICTS OF INTERESTF.-J. Neumann has received lecture fees, paid to his institution, from Amgen, Daiichi-Sankyo, Novartis, and Ferrer; has received lecture fees, paid to his institution, and consulting fees, paid to his institution, from AstraZeneca and Boehringer Ingelheim; has received grant support and lecture fees, paid to his institution, from Pfizer, Biotronic, Edwards Lifesciences, Bayer HealthCare, and GlaxoSmithKline; and has received grant support from Medtronic, Abbott Vascular, and Boston Scientific. D. Sibbing has received personal fees from Daiichi Sankyo, Sanofi and AstraZeneca, Bayer, Pfizer and Servier. I. Bernlochner has received lecture fees from Sysmex Europe GmbH. D.J. Angiolillo has received payment as an individual for: a) consulting fee or honorarium from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company; b) participation in review activities from CeloNova and St Jude Medical. Institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co, Merck, Novartis, Osprey Medical, Renal Guard Solutions and the Scott R. MacKenzie Foundation. W. Hochholzer has received consulting fees from Daiichi-Sankyo, the Medicines Company; has received lecture fees from Bayer Vital, Boehringer, Bristol-Myers Squibb and AstraZeneca. S. Kufner reports personal fees from Bristol-Myers Squibb, AstraZeneca, and Translumina. H.B. Sager has received funding from the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme (STRATO), the Else-Kröner-Fresenius-Stiftung, and the Deutsche Forschungsgemeinschaft. M. Joner has received personal fees from Abbott, Biotronik, Orbus Neich, Boston Scientific, Edwards, AstraZeneca, Schockwave and Recor; and has received grants from Boston Scientific, Edwards, Cardiac Dimensions, InfraRedx, and Amgen. H. Schunkert has received personal fees from Merck Sharp & Dohme, Amgen, Bayer Vital GmbH, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, Servier, Brahms, Bristol-Myers Squibb, Medtronic, Sanofi, Synlab; and has received grants and personal fees from AstraZeneca. S. Schüpke has received the Else Kröner-Memorial grant from the Else Kröner-Fresenius Stiftung, financial support from the German Center for Cardiovascular Research (DZHK) and consulting fees from Bayer Vital GmbH and lecture fees from Daiichi-Sankyo and Biopas Laboratories. A. Akin has received lecture fees from Boston Scientific, Boehringer, Daiichi-Sankyo, Gore. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.11.007