Brugada syndrome (BS) is an inherited channelopathy associated with elevated risk of ventricular fibrillation and sudden cardiac death. Its diagnosis is based on a standard electrocardiographic pattern comprising ST-segment elevation ≥ 2 mm in right precordial leads (V1 and V2), followed by a negative T wave (type 1 Brugada pattern). This pattern can be spontaneously observed or induced by fever or a provocation test involving sodium channel blockers. However, certain drugs and conditions (eg, electrolyte imbalances, myocardial ischemia) can induce type 1 Brugada pattern in individuals without the congenital syndrome.1 Riera et al.2 named this condition “Brugada phenocopy” (BrP) when it meets a series of diagnostic criteria3: an electrocardiographic pattern compatible with types 1 or 2 Brugada, a plausible cause, normalization of the electrocardiographic pattern after its resolution, low probability of BS, absence of compatible symptoms and personal and family history, a negative genetic study, and a negative provocation test with sodium channel blockers.
In May 2020, reports began to appear of children who developed, weeks after acute SARS-CoV-2 infection, a multisystem inflammatory syndrome of variable expressivity that was named pediatric multisystem inflammatory syndrome related to SARS-CoV-2 (SIM-PedS).4 These symptoms can present as complete or incomplete Kawasaki disease and in conjunction with gastrointestinal symptoms, shock, hypotension, and myocardial dysfunction.
The cardiac involvement of SARS-CoV-2 can include electrocardiographic changes compatible with BrP. However, no “confirmed” phenocopy has been described in this context.
We present the case of a 12-year-old boy admitted with a 4-day history of abdominal pain, diarrhea, vomiting, and 40°C fever. At admission, he had blood pressure of 74/41mmHg (< 5th percentile for his age and weight), tachycardia, and normal oxygen saturation. His overall condition was poor on examination, with cracked lips, conjunctival hyperemia, and strawberry tongue. Blood tests showed elevations in inflammatory markers (C-reactive protein, 32mg/dL), with NT-proBNP at 14 700 pg/mL and troponin I at 1581 ng/L. Serological tests were positive for immunoglobulin G for SARS-CoV-2. The patient met clinical and analytical criteria compatible with SIM-PedS.
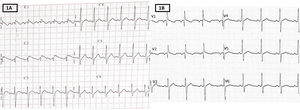
Electrocardiography (figure 1A) revealed type 1 Brugada pattern. Echocardiography showed a left ventricular ejection fraction of 55%, with no other abnormalities.
Given his poor hemodynamic improvement, with blood pressure consistently less than the 5th percentile for his age, he was admitted to the pediatric intensive care unit for vasoactive support. In addition, treatment against SIM-PedS was administered, comprising intravenous immunoglobulin, aspirin, and methylprednisolone, which improved the clinical situation and gradually reduced the levels of NT-proBNP and troponin. The electrocardiographic abnormalities resolved before hospital discharge (figure 1B).
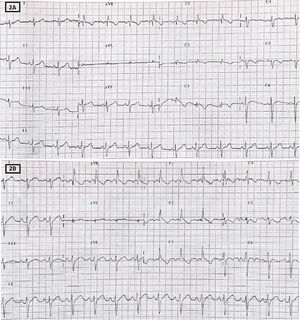
During follow-up in the pediatric cardiology clinic and to rule out the diagnosis of BS, we expanded the patient and family study. There was no family history suggestive of inherited channelopathy. Electrocardiography in the Brugada position ruled out the presence of this pattern in the patient's parents. A genetic study targeting mutations associated with BS, involving 21 genes, obtained no abnormal findings. Finally, drug provocation testing was performed with flecainide (figure 2), which failed to reveal a concealed Brugada pattern. The patient's legal guardians provided informed consent for the publication of the case.
Cardiac involvement is very common in SIM-PedS. A study of 286 cases of SIM-PedS from 17 European countries5 showed that the most common cardiovascular complications were shock, cardiac arrhythmias, pericardial effusion, and coronary dilatation. Almost half of the patients had reduced ejection fraction and most had troponin elevation, as well as an increase in inflammatory parameters. Electrocardiographic abnormalities were seen in 35.3% of patients at admission; the most common were repolarization abnormalities (ST-segment or T wave) and a prolonged PR interval. However, only one other case has been published of a pediatric patient with SIM-PedS associated with SARS-CoV-2 and with electrocardiographic evidence of type 1 Brugada pattern.6 Nonetheless, that study was not expanded to rule out BS. Thus, it cannot be considered to be BrP because it did not meet the above-mentioned diagnostic criteria.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSR. Santiago-Cortés is the lead author of the article. M. Clavero Adell and A. Ayerza Casas contributed to the article revision and supervision. D. Palanca Arias, L. Jiménez Montañés, and M. López Ramón worked on the patient's diagnosis and management and also contributed to the drafting of this article.
CONFLICTS OF INTERESTNone.