Diuretic resistance is a common problem during hospitalization and is associated with increased mortality and risk of rehospitalization.1 European Guidelines recommend a second diuretic when an adequate diuretic response is not achieved with loop diuretics.2 It remains unclear which combination therapy is the most effective.2 We aimed to evaluate the early effect and safety of chlorthalidone and acetazolamide in a cohort of patients with acute heart failure (AHF). We performed an observational, retrospective, single-center study of AHF patients and poor diuretic response defined as persistent congestion assessed by the composite congestion score and no loss of at least 1kg the previous day. Acetazolamide or chlorthalidone was added at the discretion of the treating physician. Vital signs, body weight, and diuresis on “day 0” (before the administration of the second diuretic) and on “day 1” (24hours after) were recorded. Urine and blood tests were obtained. As endpoints, we evaluated the proportion of patients with loss of at least 1kg 24hours after the second diuretic, as this was a common clinical target of successful decongestion in our clinical practice. Other endpoints were changes in weight, urinary output, natriuresis, and renal and electrolyte variations.
Continuous variables are presented as the median [interquartile range]. Categorical variables are described as absolute and relative frequencies. Comparison of clinical characteristics was analyzed by the chi-square test for categorical variables and the Mann-Whitney U test for continuous variables. Inverse-probability-weighted (IPW) regression adjustment analysis was performed to estimate the average treatment effect (ATE) of the second diuretic administration on weight loss>1kg. We obtained the propensity to receive either chlorthalidone or acetazolamide based on sex, chronic kidney disease, pulmonary disease, left and right ventricular dysfunction, and baseline creatinine. Second diuretic administration ATE was estimated based on the IPW and the baseline weight, baseline glomerular filtration rate, systolic blood pressure, and natriuresis. Differences in diuresis and natriuresis were assessed through an ANCOVA approach, adjusting for the same above-mentioned parameters, showing the effect size and the corresponding 95%. A 2-tailed P value<.05 was considered significant.
The present study conforms to the principles of the Declaration of Helsinki. Approval from the local ethics committee/internal review board was obtained and patients signed informed consent forms.
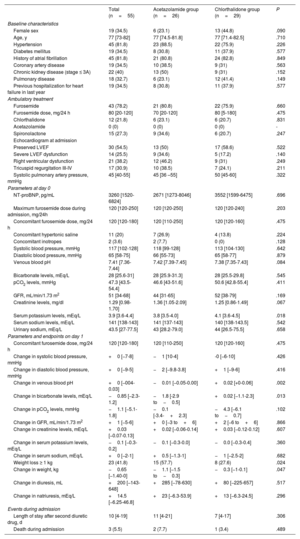
From November 2019 to March 2022, 55 patients were included. The baseline characteristics are presented in Table 1. Twenty-six patients (47.3%) were treated with acetazolamide (median dose: 125 [125-250] mg) and 29 (52.7%) with chlorthalidone (median dose: 25 [25-25] mg). The median maximum dose of furosemide during admission was 125 [120-250] mg. Combination therapy was started on the 7th day of admission (acetazolamide on the 7th [4-11 day], chlorthalidone on the 7th [5-10] day; P=.793), with a concomitant median furosemide dose of 120 [120-180] mg. Patients who received acetazolamide had lower baseline serum potassium levels.
Baseline characteristics, treatment, parameters on day 0 and day 1, length of stay and events during admission
| Total (n=55) | Acetazolamide group (n=26) | Chlorthalidone group (n=29) | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Female sex | 19 (34.5) | 6 (23.1) | 13 (44.8) | .090 |
| Age, y | 77 [73-82] | 77 [74.5-81.8] | 77 [71.4-82.5] | .710 |
| Hypertension | 45 (81.8) | 23 (88.5) | 22 (75.9) | .226 |
| Diabetes mellitus | 19 (34.5) | 8 (30.8) | 11 (37.9) | .577 |
| History of atrial fibrillation | 45 (81.8) | 21 (80.8) | 24 (82.8) | .849 |
| Coronary artery disease | 19 (34.5) | 10 (38.5) | 9 (31) | .563 |
| Chronic kidney disease (stage ≤ 3A) | 22 (40) | 13 (50) | 9 (31) | .152 |
| Pulmonary disease | 18 (32.7) | 6 (23.1) | 12 (41.4) | .149 |
| Previous hospitalization for heart failure in last year | 19 (34.5) | 8 (30.8) | 11 (37.9) | .577 |
| Ambulatory treatment | ||||
| Furosemide | 43 (78.2) | 21 (80.8) | 22 (75.9) | .660 |
| Furosemide dose, mg/24 h | 80 [20-120] | 70 [20-120] | 80 [5-180] | .475 |
| Chlorthalidone | 12 (21.8) | 6 (23.1) | 6 (20.7) | .831 |
| Acetazolamide | 0 (0) | 0 (0) | 0 (0) | - |
| Spironolactone | 15 (27.3) | 9 (34.6) | 6 (20.7) | .247 |
| Echocardiogram at admission | ||||
| Preserved LVEF | 30 (54.5) | 13 (50) | 17 (58.6) | .522 |
| Severe LVEF dysfunction | 14 (25.5) | 9 (34.6) | 5 (17.2) | .140 |
| Right ventricular dysfunction | 21 (38.2) | 12 (46.2) | 9 (31) | .249 |
| Tricuspid regurgitation III-IV | 17 (30.9) | 10 (38.5) | 7 (24.1) | .211 |
| Systolic pulmonary artery pressure, mmHg | 45 [40-55] | 45 [36 –55] | 50 [45-60] | .322 |
| Parameters at day 0 | ||||
| NT-proBNP, pg/mL | 3260 [1520-6824] | 2671 [1273-8046] | 3552 [1599-6475] | .696 |
| Maximum furosemide dose during admission, mg/24h | 120 [120-250] | 120 [120-250] | 120 [120-240] | .203 |
| Concomitant furosemide dose, mg/24 h | 120 [120-180] | 120 [110-250] | 120 [120-160] | .475 |
| Concomitant hypertonic saline | 11 (20) | 7 (26.9) | 4 (13.8) | .224 |
| Concomitant inotropes | 2 (3.6) | 2 (7.7) | 0 (0) | .128 |
| Systolic blood pressure, mmHg | 117 [102-128] | 118 [99-128] | 113 [104-130] | .642 |
| Diastolic blood pressure, mmHg | 65 [58-75] | 66 [55-73] | 65 [58-77] | .879 |
| Venous blood pH | 7.41 [7.36-7.44] | 7.42 [7.39-7.45] | 7.38 [7.35-7.43] | .084 |
| Bicarbonate levels, mEq/L | 28 [25.6-31] | 28 [25.9-31.3] | 28 [25.5-29.8] | .545 |
| pCO2 levels, mmHg | 47.3 [43.5-54.4] | 46.6 [43-51.6] | 50.6 [42.8-55.4] | .411 |
| GFR, mL/min/1.73 m2 | 51 [34-68] | 44 [31-65] | 52 [38-79] | .169 |
| Creatinine levels, mg/dl | 1.29 [0.98-1.70] | 1.36 [1.05-2.09] | 1.25 [0.86-1.49] | .067 |
| Serum potassium levels, mEq/L | 3.9 [3.6-4.4] | 3.8 [3.5-4.0] | 4.1 [3.6-4.5] | .018 |
| Serum sodium levels, mEq/L | 141 [138-143] | 141 [137-143] | 140 [138-143.5] | .542 |
| Urinary sodium, mEq/L | 43.5 [27-77.5] | 43 [28.2-79.0] | 44 [26.5-75.5] | .658 |
| Parameters and endpoints on day 1 | ||||
| Concomitant furosemide dose, mg/24 h | 120 [120-180] | 120 [110-250] | 120 [120-160] | .475 |
| Change in systolic blood pressure, mmHg | +0 [−7-8] | −1 [10-4] | -0 [−6-10] | .426 |
| Change in diastolic blood pressure, mmHg | +0 [−9-5] | −2 [−9.8-3.8] | +1 [−9-6] | .416 |
| Change in venous blood pH | +0 [−004-0.03] | −0.01 [−0.05-0.00] | +0.02 [+0-0.06] | .002 |
| Change in bicarbonate levels, mEq/L | −0.85 [−2.3-1.2] | −1.8 [-2.9 to−0.5] | +0.02 [−1.1-2.3] | .013 |
| Change in pCO2 levels, mmHg | −1.1 [−5.1-1.8] | −0.1 [-3.4-+2.3] | −4.3 [−6.1 to−0.7] | .102 |
| Change in GFR, mL/min/1.73 m2 | +1 [−5-6] | +0 [−3 to+6] | +2 [−6 to+6] | .866 |
| Change in creatinine levels, mEq/L | +0.03 [−0.07-0.13] | +0.02 [−0.06-0.14] | +0.03 [−0.12-0.12] | .607 |
| Change in serum potassium levels, mEq/L | −0.1 [−0.3-0.2] | −0.1 [−0.3-0.0] | −0.0 [−0.3-0.4] | .360 |
| Change in serum sodium, mEq/L | +0 [−2-1] | +0.5 [−1.3-1] | −1 [−2.5-2] | .682 |
| Weight loss ≥ 1 kg | 23 (41.8) | 15 (57.7) | 8 (27.6) | .024 |
| Change in weight, kg | −0.65 [−1.40-0] | −1.1 [−1.5 to−0.3] | −0.3 [−1-0.1] | .047 |
| Change in diuresis, mL | +200 [−143-648] | +285 [−78-630] | +80 [−225-657] | .517 |
| Change in natriuresis, mEq/L | +14.5 [−6.25-46.8] | +23 [−6.3-53.9] | +13 [−6.3-24.5] | .296 |
| Events during admission | ||||
| Length of stay after second diuretic drug, d | 10 [4-19] | 11 [4-21] | 7 [4-17] | .306 |
| Death during admission | 3 (5.5) | 2 (7.7) | 1 (3.4) | .489 |
GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
The data are expressed as No. (%) or median [interquartile range].
Overall, 41.8% of the patients lost at least 1kg after the association of a second diuretic. After adjustment through the IPW, the average treatment effect of acetazolamide vs chlorthalidone was 0.36 (95% confidence interval [95%CI], 0.09-0.63, P=.008). The estimated potential-outcome mean of losing ≥ 1kg in the patients who received acetazolamide was 62% (95%CI, 39-84) and 26% (95%CI, 10-41) for those receiving chlorthalidone.
There were no significant differences in diuresis (effect size of acetazolamide:+202.5mL, 95%CI,−155.1-560.1; P=.260) and natriuresis (effect size of acetazolamide:+13.11 mEq/l (95%CI,−2.35-28.59, P=.095).
The use of acetazolamide was associated with a greater decrease in pH and bicarbonate levels, but the magnitude of the difference was low.
To date, there are no valuable parameters to predict in-hospital diuretic resistance beyond urinary sodium after the first diuretic administration,3 but the value of natriuresis after several days remains unclear.3 It is important to note that our patients received the coadjuvant diuretic on the 7th day of admission.
On the other hand, although weight change may be influenced by water-food intake, its measurement is commonly used to monitor response to diuretic therapy. Several studies have shown that a poor diuretic response assessed by weight is associated with a worse prognosis.1 In clinical practice, the goal of a daily loss of 1kg while congestion persists seems reasonable.
European Guidelines recommend a combined diuretic strategy based on sequential nephron blockade, suggesting thiazides as the first-line treatment and acetazolamide as second-line.2 These recommendations were based on their pathophysiological mechanisms and observational studies. Two randomized, placebo-controlled trials, CLOROTIC4 and ADVOR,5 have recently shown the efficacy of hydrochlorothiazide and acetazolamide, respectively, in obtaining successful decongestion when added to a loop diuretic. To date, no randomized trials have compared the use of oral thiazides with acetazolamide in combination with loop diuretics.2 To our knowledge, the CANDI trial is the first to evaluate the early effect of oral acetazolamide and chlorthalidone on top of loop diuretics in patients admitted for AHF with diuretic resistance. With the limitations of a single-center, observational study, we found that compared to chlorthalidone, acetazolamide was associated with a higher proportion of patients with 24-hour weight loss of at least 1kg with a neutral effect on 24-hour diuresis and natriuresis.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSM. Cobo Marcos contributed to the conception and design of the study. D. Sánchez Ortiz and A. Matutano Muñoz contributed to patient inclusion. P. Vela Martín contributed to data inclusion in the database. P. Vela Martín organized the database. A. Royuela performed the statistical analysis. P. Vela Martín, M. Cobo Marcos and F. Domínguez wrote the first draft of the manuscript. All authors contributed to manuscript revision, and have read and approved the submitted version
CONFLICTS OF INTERESTSNone.
.