Transthyretin cardiac amyloidosis (ATTR-CA) patients often have atrial fibrillation and increased bleeding/thrombogenic risks. We aimed to evaluate outcomes of left atrial appendage closure (LAAC) compared with patients without a known diagnosis of CA.
MethodsComparison at long-term of patients diagnosed with ATTR-CA who underwent LAAC between 2009 and 2020 and those without a known diagnosis of CA.
ResultsWe studied a total of 1159 patients. Forty patients (3.5%) were diagnosed with ATTR-CA; these patients were older and had more comorbidities, higher HAS-BLED and CHA2DS2-VASc scores, and lower left ventricular function. Successful LAAC was achieved in 1137 patients (98.1%) with no differences between groups. Regarding in-hospital and follow-up complications, there were no differences between the groups in ischemic stroke (5% vs 2.5% in those without a known diagnosis of CA; P=.283), hemorrhagic stroke (2.5% and 0.8% in the control group; P=.284), major or minor bleeding. At the 2-year follow-up, there were no significant differences in mortality (ATTR-CA: 20% vs those without known CA: 13.6%, 0.248); however, the at 5-year follow-up, ATTR-CA patients had higher mortality (40% vs 19.2%; P <.001) but this difference was unrelated to hemorrhagic complications or ischemic stroke.
ConclusionsLAAC could reduce the risk of bleeding complications and ischemic cerebrovascular events without increasing the rate of early or mid-term complications. Although long-term survival was impaired in ATTR-CA patients, it was comparable to that of patients without a known diagnosis of CA at the 2-year follow-up, suggesting that LAAC for patients with ATTR-CA might not be futile.
Keywords
Cardiac amyloidosis (CA) is a cardiomyopathy caused by extracellular deposition of unstable structured proteins in the myocardium giving rise to restrictive physiology in the heart.1 Around 98% of cases are due to deposition of fibrils composed of monoclonal immunoglobulin light chains (AL) or transthyretin amyloidosis (ATTR), but there are 9 amyloidogenic proteins that can affect the myocardium.2 Diagnosis of CA begins with a phase of suspicion (recognizing red flags) and ends in a definitive diagnosis based on invasive or noninvasive criteria.1,2 Nevertheless, only transthyretin cardiac amyloidosis (ATTR-CA) can be diagnosed without invasive criteria (biopsy) when there are supporting cardiac imaging findings, Peruggini grade 2 or 3 radiotracer myocardial uptake on scintigraphy, and absence of other clonal dyscrasia.2,3 After confirmation of CA, prognosis is variable and depends on disease stage and the type of CA (AL-CA, wild-type ATTR or hereditary-type ATTR); 5-year survival varies between 14% and 68%.4,5 Of note, the prevalence and incidence rates of ATTR-CA have constantly increased since 2000 and it is now the most frequently diagnosed type, affecting particularly older men in the case of wild-type ATTR and affecting patients with polyneuropathy and cardiomyopathy in the case of hereditary forms.4–6
During the course of the disease, several arrhythmic manifestations can occur, including nonspecific conduction disturbances, bradyarrhythmias, atrial fibrillation (AF), and sudden cardiac arrest.7 The prevalence of AF in ATTR-CA is around 70% in wild-type ATTR and is 54% in hereditary-type ATTR, and it is therefore the most common arrhythmia associated with this disease.8,9 Even when AF does not seem to increase mortality in this setting, it can increase heart failure episodes, and its treatments, based on antiarrhythmic drugs and anticoagulation, are not always well tolerated by patients with CA.7,10 Moreover, bleeding and thrombogenic risks are higher than estimated by most scores.11 Some strategies such as rhythm control or ablation are controversial as a therapeutic option for the treatment of AF in these patients, but thrombotic risk remains high even when anticoagulation is used and probably despite rhythm control.12,13 In addition, although left atrial appendage closure (LAAC) has proven good results in patients with AF and high bleeding risk,14 this procedure has not been studied in the CA population, who have shortened life-expectancy; therefore, its indication in this setting remains unclear given that procedural risks might be increased and its efficacy in preventing thrombus formation is unclear.
The aim of this study was to evaluate whether LAAC in patients with AF and ATTR-CA had similar outcomes to patients without a known diagnosis of CA.
METHODSStudy populationRetrospective study including consecutive patients with AF who underwent LAAC due to contraindication of anticoagulation between December 2009 and December 2020 across 11 Spanish hospitals. Patients diagnosed with ATTR-CA were retrospectively reviewed and the diagnostic criteria of the Working Group on Myocardial and Pericardial Diseases of the European Society of Cardiology2 were verified when possible; patients were compared with those without an ATTR-CA diagnosis (control group). The decision to exclude other types of CA was taken prior to data collection, but in the end, none was found in the study population. Of note, CA was not routinely investigated in the control group. All clinical data, in-hospital and follow-up outcomes were prespecified in the online database used by the centers, complying with the requirements of the Law on Data Protection, and were accessible only to participating operators and registry coordinators. Follow-up was retrospectively performed by review of clinical records and direct telephone contact when clinical records were incomplete or missing. This study was approved by local ethics committees.
Study endpointsThe primary endpoint was to compare in-hospital and follow-up outcomes between patients with ATTR-CA and the control group who underwent LAAC. Secondary endpoints were to compare follow-up mortality between the 2 groups, to describe the clinical features of the ATTR-CA population and to define mortality predictors. Futility was defined as survival of less than 1-year after the procedure.
Statistical analysisCategorical variables are presented as frequencies, and comparisons between groups were performed using the chi-square or Fisher exact test when necessary. Continuous variables are expressed as mean (± standard deviation) or median [25th-75th interquartile range]. The normal distribution of continuous variables was tested using the Kolmogorov-Smirnov test, and graphically tested using the Q-Q plot. Between-group comparisons were performed using the t test or Mann-Whitney U test according to the distribution of the variables.
Cox multivariable regression analysis was performed to identify the independent predictors of 2-year mortality. The multivariable model was built by backward stepwise (likelihood ratio) selection. Variables associated with mortality in the univariate analysis were included in the model. Verification of the proportional hazard assumption was performed. The variables included were age, body surface area, left ventricular ejection fraction, diabetes, previous coronary disease, previous ischemic stroke, previous hemorrhagic stroke, chronic kidney disease, liver disease, the presence of ATTR-CA, and CHA2DS2-VASc scores. In addition, the incidence of ischemic and bleeding complications was recorded in hospital and at long-term and was analyzed independently and as a combined endpoint including both ischemic and hemorrhagic cerebrovascular events.
We analyzed time to 2- and 5-year mortality by Kaplan-Meier event-free survival curves and comparison was performed using the log-rank test. A competing risk analysis was performed for mortality at 2 years of follow-up.15 All tests were 2 sided at the .05 significance level. All analyses were performed using R software, version 3.6.1 (R Project for Statistical Computing, Austria).
RESULTSA total of 1159 patients who underwent LAAC were included in this study. Of these, 40 patients (3.5%) were diagnosed with ATTR-CA (2 patients were diagnosed immediately after the procedure and 38 before the procedure) and 1119 (96.5%) had no evidence of the disease.
Baseline characteristics of the study populationThe main baseline characteristics are summarized in table 1. Patients in the ATTR-CA group were more often male (34 patients [85%] vs 707 patients [63.2%]; P=.005), were significantly older (83.1±4.9 vs 75.9±8.2 years; P <.001), and had lower body mass index (25.9±3.1 vs 27.2±4.5kg/m2; P=.017). They also had more comorbidities, particularly diabetes mellitus (20 patients [50%] vs 374 patients [33.4%]; P=.030), chronic kidney disease (28 patients [70%] vs 456 patients [40.8%]; P <.001), and peripheral artery disease (11 patients [27.5%] vs 153 patients [14.3%]; P=.020).
Main clinical and echocardiographic characteristics at baseline of the overall population and groups.
| Overall populationN=1159 (100%) | Control groupn=1119 (96.5%) | ATTR-CAn=40 (3.5%) | P | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Sex, female | 418 (36.1) | 412 (36.8) | 6 (15) | .005* |
| Age | 76.1±8.1 | 75.9±8.2 | 83.1±4.9 | <.001* |
| BMI | 27.2±4.4 | 27.2±4.5 | 25.9±3.1 | .017* |
| BSA | 1.83±0.21 | 1.83±0.2 | 1.85±0.16 | .512 |
| Hypertension | 963 (83.1) | 931 (83.2) | 32 (80) | .596 |
| Diabetes mellitus | 394 (34) | 374 (33.4) | 20 (50) | .030* |
| Smoker | 298 (25.7) | 295 (26.4) | 3 (7.5) | .007* |
| Alcohol consumption | 73 (6.3) | 70 (6.3) | 3 (7.5) | .736 |
| Prior coronary disease | 263 (22.7) | 258 (24.1) | 5 (12.5) | .089 |
| Prior PCI | 154 (13.3) | 151 (15.4) | 3 (7.5) | .170 |
| Prior CABG | 58 (5) | 56 (5.7) | 2 (5) | .999 |
| Prior valve surgery | 53 (4.6) | 51 (6.1) | 2 (5) | .999 |
| Peripheral artery disease | 164 (14.2) | 153 (14.3) | 11 (27.5) | .020* |
| Prior ischemic stroke | 320 (27.6) | 306 (29) | 14 (35) | .415 |
| Prior hemorrhagic stroke | 252 (21.7) | 239 (23.4) | 13 (32.5) | .185 |
| Prior TIA | 85 (7.3) | 69 (6.8) | 16 (40) | <.001* |
| Previous peripheral embolization | 31 (2.7) | 29 (3) | 2 (5) | .347 |
| CKD | 484 (41.8) | 456 (40.8) | 28 (70) | <.001* |
| Liver disease | 89 (7.7) | 87 (8.1) | 2 (5) | .765 |
| Previous bleeding | 839 (72.5) | 804 (71.9) | 35 (87.5) | .030* |
| HAS-BLED | 4 [3-4] | 3.5 [3-4] | 4 [4-4] | .001* |
| CHA2DS2-VASc | 4 [3-5] | 4.3 [3-5] | 5.2 [5-6] | <.001* |
| Type of AF | ||||
| Paroxysmal | 398 (34.3) | 393 (35.3) | 5 (12.5) | .003* |
| Persistent-permanent | 754 (65.1) | 719 (64.7) | 35 (87.5) | |
| Flutter | 50 (6.5) | 50 (6.9) | 0 (0) | .102 |
| Echocardiographic findings | ||||
| Mitral regurgitation III-IV | 34 (2.9) | 29 (2.6) | 5 (12.5) | .005* |
| LVEF | 58.1±10.7 | 58.5±10.5 | 46.8±8.7 | <.001* |
AF, atrial fibrillation; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass graft; CKD, chronic kidney disease (defined as GFR <60mL/min/1.73); LVEF, left ventricular ejection fraction; PCI: percutaneous coronary intervention; TIA, transient ischemic attack.
There were no differences in the prior stroke rate, either ischemic or hemorrhagic, but ATTR-CA patients more often had prior transitory ischemic attacks (16 patients [40%] vs 69 patients [6.8%]; P <.001) and overall bleeding events (35 patients [87.5%] vs 804 patients [71.9%]; P=.030). Patients in the amyloidosis group also had higher HAS-BLED and CHA2DS2-VASc scores (4 [4-4] vs 3.5 [3-4], P=.001 and 5.2 [5-6] vs 4.3 [3-5]; P <.001, respectively). Finally, left ventricular ejection fraction was lower (46.8±8.7 vs 58.5±10.5%; P <.001) and the mitral regurgitation (MR) rate was higher in the amyloidosis group (MR III-IV: 12.5% in ATTR-CA patients vs 2.6%; P=.005).
Specific characteristics of patients with ATTR-CA are summarized in table 2. Wild-type ATTR-CA was diagnosed in 65% of these patients (n=26), hereditary-type ATTR in 12.5% (n=5) and, in 22.5% of the patients (n=9), the type was not studied. The main symptoms associated with the disease were dyspnea in 97.5%, syncope in 17.5%, and carpal tunnel syndrome in 12.5%; but there were no cases of biceps tendon rupture or orthostatism; lumbar spinal stenosis was observed in 5% of the patients. After careful electrocardiographic review, conduction disturbances were present in 77.5% of the patients (12.5% had first-degree atrioventricular block, 52.5% left bundle branch block, 20% had right bundle branch block, and 30% had other conduction disturbances).
Specific clinical and electrocardiographic characteristics in the cardiac amyloidosis cohort.
| ATTR subtype (n=40) | |
| ATTRv | 5 (12.5) |
| ATTwt | 26 (65) |
| ATTR of unknown subtype | 9 (22.5) |
| Reported symptoms in clinical records (n=40) | |
| Dyspnea | 39 (97.5) |
| NYHA III-IV | 27 (67.5) |
| Angina | 2 (5) |
| Syncope | 7 (17.5) |
| Orthostatism | 0 |
| Biceps tendon rupture | 0 |
| Carpal tunnel syndrome | 5 (12.5) |
| Lumbar spinal stenosis | 2 (5) |
| Heart failure | 33 (82.5) |
| Electrocardiographic findings (n=40) | |
| Conduction disturbance | 31 (77.5) |
| AVB 1st | 5 (12.5) |
| LBBB | 21 (52.5) |
| RBBB | 8 (20) |
| Unspecific intraventricular disturbance | 12 (30) |
| Pseudoinfarction pattern | 4 (10) |
| Low voltage | 5 (12.5) |
| LV hypertrophy | 7 (17.5) |
| Biochemical parameters (n=38) | |
| NT-pro-BNP, pg/dL | 3615±1276 |
| GFR, mL/min | 51±21 |
| Echocardiographic findings (n=36) | |
| Maximal septal width, mm | 2 0±6 |
| Left atrial diameter (parasternal long-axis view), mm | 51±17 |
| Diagnostic method (n=40) | |
| Invasive | 9 (22.5) |
| Noninvasive (DPD-scan) | 32 (80) |
| National Amyloidosis Center classification (n=40) | |
| Stage I | 22 (55) |
| Stage II | 14 (35) |
| Stage III | 4 (10) |
ATTR, transthyretin amyloidosis; ATTRv, hereditary-type transthyretin amyloidosis; ATTwt, wild-type transthyretin amyloidosis; AVB, atrioventricular block; LBBB, left bundle branch block; LV, left ventricle; NYHA, New York Heart Association class; RBBB, right bundle branch block.
Data are expressed as No. (%) or mean±standard deviation.
The main procedural and follow-up outcomes are summarized in table 3. The main indication for LAAC in the overall population was prior bleeding under oral anticoagulation (67.1%) followed by high risk of bleeding (17.7%) and stroke despite anticoagulation (12.9%), with no differences regardless of the presence of ATTR-CA. Other indications included labile international normalized ratio or high risk of falls in 2.2%. The most commonly used device was the Amulet (46.2%) followed by the WATCHMAN device (37.4%). There were no significant differences in outcomes when the indication for closure and the device used were compared between the groups (). The proportion of patients with ATTR-CA treated with LAAC was higher after 2015 (82.5% vs 70.5% in the control group) but this difference was not statistically significant (P=.102).
Main procedural and follow-up outcomes of the overall population and according to the presence of cardiac amyloidosis.
| Overall populationN=1159 (100%) | Control groupn=1119 (96.5%) | ATTR-CAn=40 (3.5%) | P | |
|---|---|---|---|---|
| Reason for LAAC | ||||
| Prior systemic bleeding | 778 (67.1) | 747 (66.8) | 31 (77.5) | .394 |
| Stroke /embolism with OAC | 150 (12.9) | 145 (13) | 5 (12.5) | |
| High bleeding risk w/o prior bleeding | 205 (17.7) | 201 (18) | 4 (10) | |
| Other reasons (labile INR, high risk of falling, or others) | 26 (2.2) | 26 (2.3) | 0 | |
| Device | ||||
| WATCHMAN | 434 (37.5) | 423 (37.9) | 11 (27.5) | .450 |
| Amulet | 536 (46.2) | 515 (46) | 21 (52.5) | |
| ACP | 115 (9.9) | 109 (9.7) | 6 (15) | |
| Other | 74 (6.4) | 72 (6.4) | 2 (5) | |
| Procedural success | 1137 (98.1) | 1097 (98) | 40 (100) | .999 |
| Procedural complications | 48 (4.1) | 48 (4.3) | 0 | .406 |
| Cardiac tamponade | 21 (1.8) | 21 (1.9) | 0 | .999 |
| Stroke | 5 (0.4) | 5 (0.4) | 0 | .999 |
| Device embolization | 5 (0.4) | 5 (0.4) | 0 | .999 |
| Vascular complication | 9 (0.8) | 8 (0.7) | 1 (2.5) | .272 |
| Major bleeding | 8 (0.7) | 8 (0.7) | 0 | .999 |
| Procedural death | 1 (0.1) | 1 (0.1) | 0 | .999 |
| In-hospital complications | 39 (3.3) | 37 (3.5) | 2 (5) | .641 |
| In-hospital ischemic stroke | 5 (0.4) | 5 (0.5) | 0 | .999 |
| In-hospital hemorrhagic stroke | 0 (0) | 0 (0) | 0 | .999 |
| In-hospital ischemic +hemorrhagic stroke | 5 (0.4) | 5 (0.5) | 0 | .999 |
| In-hospital TIA | 3 (0.3) | 3 (0.3) | 0 | .999 |
| In-hospital peripheral embolism | 0 | 0 | 0 | .999 |
| In-hospital major bleeding | 19 (1.6) | 19 (1.7) | 0 | .999 |
| In-hospital minor bleeding | 14 (1.2) | 12 (1.1) | 2 (5) | .081 |
| In-hospital death | 6 (0.5) | 6 (0.5) | 0 | .999 |
| Follow-up | ||||
| Mean follow-up, d | 1311±215 | 1339±221 | 1301±202 | .999 |
| FU ischemic stroke | 29 (2.5) | 27 (2.5) | 2 (5) | .283 |
| FU hemorrhagic stroke | 9 (0.8) | 8 (0.8) | 1 (2.5) | .284 |
| FU ischemic + hemorrhagic stroke | 38 (3.3) | 35 (3.1) | 3 (7.5) | .127 |
| FU TIA | 8 (0.7) | 8 (0.8) | 0 | .999 |
| FU peripheral embolism | 9 (8) | 9 (0.8) | 0 | .999 |
| FU major bleeding | 84 (7.2) | 81 (7.2) | 3 (7.5) | .999 |
| FU minor bleeding | 77 (6.6) | 76 (6.9) | 1 (2.5) | .514 |
| Type of antithrombotic therapy | ||||
| No antithrombotics | 96 (8.3) | 74 (6.6) | 12 (30) | <.001* |
| FU single antiplatelet therapy | 851 (73.4) | 839 (75) | 22 (55) | |
| FU dual antiplatelet therapy | 69 (5.9) | 69 (6.2) | 0 | |
| FU anticoagulation | 143 (12.3) | 137 (12.2) | 6 (15) | |
| 2-year FU death | 160 (13.8) | 152 (13.6) | 8 (20) | .248 |
| 5-year FU death | 231 (19.9) | 215 (19.2) | 16 (40) | .001 |
FU, follow-up; LAAC, left atrial appendage closure; OAC, oral anticoagulation; w/o, without; TIA, transient ischemic attack.
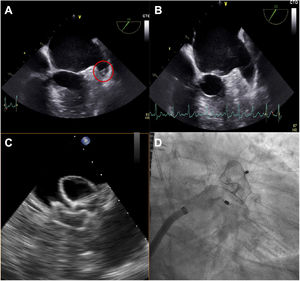
During the procedure, the presence of thrombi in the left atrial appendage was detected by transoesophageal echocardiography in 7.5% of the patients with ATTR-CA (vs 2.9% in patients without amyloidosis; P=.241) (figure 1), leading to a delay of the procedure in 93.3% of them and its performance with cerebral protection devices in 2.7% of the overall cohort; one case of device-related thrombosis was detected in the control group at follow-up according to the criteria previously described.16
Case example of ATTR cardiac amyloidosis presenting a thrombus during the first attempt at left atrial appendage closure (A); after 1 month of subcutaneous low-weight heparin, the thrombus disappeared (B) and the procedure could be successfully performed (C: echocardiographic guidance of deployment; D: angiographic result).
Successful LAAC was achieved in 98.1% of the overall population, with a similar success rate in the 2 groups (100% in the amyloidosis group vs 98% in the control group; P=.999). Overall, the rate of acute procedural complications (including tamponade, stroke, device embolization, vascular complications, and any bleeding) was 4.1% without significant differences between the groups. During in-hospital stay, all outcomes were comparable except for a trend to more minor bleeding events related to the access site in the ATTR-CA group (5% vs 1.1%, P=.081). In-hospital mortality was 0.5% in the overall population, but no differences were found between groups (0.5% in the control group vs 0% in the amyloidosis group; P=.999).
Follow-up outcomesIn the follow-up, there was a nonsignificant higher rate of both ischemic and hemorrhagic cerebrovascular events in ATTR-CA patients; the combined endpoint of all-cause stroke remained higher in ATTR-CA patients.
At short-term follow-up (median of 3 [2-3] months postprocedure), most patients in the 2 groups received antiplatelet monotherapy (55% in ATTR-CA patients vs 75% in the control group) but none of the patients with CA received dual antiplatelet therapy (vs 6.2%) and 30% did not receive any antithrombotic therapy at discharge (vs 6.6% in patients free of CA); P <.001 for all.
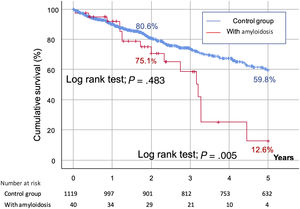
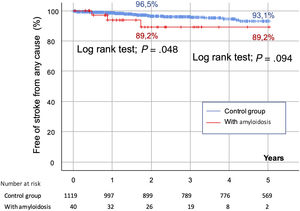
As reflected in figure 1 of the supplementary data, futility, defined as mortality within the first year postprocedure, was comparable in the ATTR-CA and the control groups (< 10%). At the 2-year follow-up, the mortality rate was higher in the amyloidosis group (20% vs 13.6% in the control group), although this difference was not statistically significant (P=.248) (figure 2). However, at the 5-year follow-up, the mortality rate was higher in CA patients (40% vs 19.2%; P=.001) (figure 2). According to time of diagnosis of CA, patients in the first tertile (n=11, 5-year mortality: 45.4%) showed similar outcomes to those in the second (n=14, mortality: 42.8%) and third tertiles (n=15, mortality: 33.3%) (P=.310, nonsignificant). There were no differences in the rates of hemorrhagic stroke (subdistribution hazard ratio [SHR], 3.483 [0.450-26.956]; P=.232), major bleeding (SHR, 0.646 [0.161-2.599]; P=.539), ischemic stroke (SHR, 2.035 [0.468-8.840], P=.343), or peripheral embolism (SHR, 2.459 [0.814-5.622]; P=.354), or for the combination of these 4 events at 5 years of follow-up (SHR, 2.379 [0.723-7.827]; P=.154). Survival curves at 5 years of follow-up for all-cause cerebrovascular events are shown in figure 3, suggesting a higher rate of all-cause stroke in the CA group at the 2-year follow-up. The main final cause of mortality in the CA group is described in detail in . CA was not an independent predictor of mortality at the 2-year follow-up () and this finding was not modified by the competing risk analysis ().
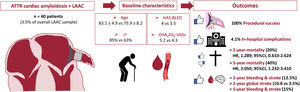
This is the first study to systematically examine the acute and long-term outcomes of patients with ATTR-CA requiring LAAC. The main findings, summarized in figure 4, are as follows: a) even across patients referred for LAAC, who have multiple comorbidities, there was a remarkably poorer profile in those diagnosed with ATTR-CA, particularly in terms of bleeding and thrombotic risk; b) despite the frailty of patients with ATTR-CA, procedural and in-hospital outcomes were good with only a relatively higher rate of site-related minor bleeding events postprocedurally that did not have an impact on mortality at mid-term; c) outcomes at the 2-year follow-up did not differ much, although the substantial differences in percentages might suggest that the study was underpowered to make strong claims about differences in mortality; nevertheless, it supports the conclusion that the indication for LAAC can be established as being similar to that in patients without this condition since it is similarly safe and probably not futile. The high mortality rate in patients with CA at the 5-year follow-up is in agreement with prior series of ATTR-CA but in most patients was not related to hemorrhagic or embolic events likely due to a prior successful LAAC procedure.
The safety of the LAAC procedure is more important than for other interventional procedures, given its preventive nature and the frailty of patients undergoing the technique. This might be particularly relevant if CA is present since most of the baseline features in these patients (age, lower body mass index, chronic kidney disease) are related to an increased rate of several complications including procedural bleeding.
Moreover, the CHA2DS2-VASc and HAS-BLED scores might not be as accurate as in the general population. Indeed, in previous research, autopsies revealed that 33% of hearts with amyloidosis had intracardiac thrombi irrespective of the presence of traditional risk factors such as age, hypertension, diabetes, or heart failure.11 For this reason, chronic anticoagulation is indicated in CA patients with AF irrespective of their scores. For the same reason, and given the safety of the procedure reported in this study, LAAC could be considered in this population in patients with embolic events despite anticoagulation or in those with frequent bleeding events while receiving oral anticoagulation irrespective of the score. The fact that most patients were in National Amyloidosis Center stage I when diagnosed implies longer expected survival, which translates into more complex decisions about thrombotic and hemorrhagic risks in which LAAC could be a key tool.
Regarding bleeding risk, several studies suggest that bleeding events occur more often in AL amyloidosis than estimates given by traditional scores; in ATTR-CA patients, there are no studies leading to the same conclusion but patients with this condition are usually older and have several cardiovascular risk factors that might play a synergistic role with ATTR-CA and lead traditional bleeding scores to also underestimate the true bleeding risk according to experts.17,18
The optimal procedural outcomes reported in this work, with just 2.5% of procedural complications, is in agreement with similar reports in standard LAAC candidates (eg, a 2.1% procedural-related complication rate in the PRAGUE-17 trial).19 These results, together with adequate survival at the 2-year follow-up suggest that LAAC is probably not futile in CA patients. Indeed, it might represent a particularly useful tool for the management of the complications related to their disease. Impaired mobility, including lumbar spinal stenosis (12.5%) and an increased risk of syncope (17.5%) due to conduction disturbances (77.5%) suggest a high risk of falls that, together with a very high proportion of patients with AF and therefore requiring oral anticoagulation, represent a high-risk combination where LAAC could be a valid alternative. Taking into consideration our limited sample, minor bleeding complications, despite being significantly higher in the CA-ATTR group, could have been underestimated.
Long-term outcomes in ATTR-CA patientsLife expectancy in patients with CA is reduced. At the time of diagnosis, it has been suggested to be around 20 months for wild-type ATTR-CA if detected at an advanced stage, but the current reported overall median survival is 3.5 years for wild-type ATTR-CA, 2.6 years for hereditary-type ATTR,5,20 and 6 to 12 months for AL amyloidosis at an advanced stage.21,22 Conversely, the growing rate of early diagnosis of this disease, due to better diagnostic tools but also due to a growing aging population, suggests that dedicated analysis of the outcomes of different interventions in contemporary cohorts is crucial.23 For many years, structural heart diseases in the setting of CA were denied invasive management, but some pharmacological and percutaneous techniques have recently demonstrated prognostic benefits for these patients; the paradigms are tafamidis and transcatheter aortic valve replacement, respectively.24 However, we cannot assume that other procedures with lower prognostic gain, such as LAAC, are equally useful and, for this reason, our findings help to shed light on the best management for these patients. Demonstrating that LAAC provides benefits for up to 2 years of follow-up, even if the combined event of all-cause stroke remains higher in ATTR-CA patients, can help treating physicians in the clinical decision-making process. However, patients should first understand the bleak prognosis at 5 years of follow-up and consider conservative management at their own discretion, emphasizing that mortality in CA is mainly associated with heart failure, sudden cardiac death, and myocardial infarction. Of note, the analysis of the combined event of mortality, all-cause stroke, and bleeding events at 2 years of follow-up () confirms that amyloidosis is not a predictor of the combined endpoint by itself; however, the presence of prior chronic kidney disease (likely a consequence of the same disease) has an independent effect in this combined event and might be a marker of futility of the technique.
LimitationsThe main limitation of this study is that the presence of CA was not systematically examined in all included patients and therefore it is unknown how many of those in the control group could have had CA. In addition, this is a relatively reduced cohort of patients with ATTR-CA but still represents the largest series of patients with this condition treated with LAAC devices and with a control group without a known diagnosis of CA who underwent the procedure in the same institutions. Moreover, although the diagnostic criteria might have varied during the study, we followed a standard definition, as described in the methods. Finally, given that this is a highly specific subgroup of LAAC patients, the clinical, echocardiographic, and electrocardiographic findings cannot be extrapolated to all patients with CA.
CONCLUSIONSLAAC is a reasonable alternative to reduce bleeding complications and ischemic cerebrovascular events without an increased rate of early or mid-term complications. Although long-term survival was impaired in ATTR-CA patients, there were no significant differences compared with the control group up to the 2-year follow-up, suggesting that LAAC for patients with ATTR-CA might not be futile.
FUNDINGNone to declare.
AUTHORS’ CONTRIBUTIONSJ. R. Delgado-Arana, I. Cruz-Gonzalez, H. Gutiérrez, I. García-Bolao, X. Millán, G. Tirado-Conte, J. M. Ruiz-Nodar, M. Mohandes, J. Palazuelos, F. Torres Saura, R. Del Valle, E. Valero Picher, and I. J. Amat-Santos contributed with planning the research.
J. R. Delgado-Arana, J. C. Nuñez García, I. Gómez, R. Albarrán Rincón, D. Arzamendi, L. Nombela-Franco, L. Korniiko, A. Barrero, S. Santos-Martínez, A. Serrador, and I. J. Amat-Santos conducted the research.
J. R. Delgado-Arana and I. J. Amat-Santos drafted and wrote the final manuscript and are responsible for its overall content.
CONFLICTS OF INTERESTI. Cruz-Gonzalez is proctor for Abbott Vascular, Boston Scientific and Lifetech, J. M. Ruiz-Nodar and I. J. Amat-Santos are proctors for Boston Scientific. L. Nombela-Franco and D. Arzamendi are proctors for Abbott Vascular.
The prevalence and incidence rates of transthyretin cardiac amyloidosis (ATTR-CA) show that it has become the most commonly diagnosed type of CA. Despite the high rate of AF (54%-70%), requiring a low threshold for starting oral anticoagulation, and the high risk of bleeding complications and stroke, the risk/benefit analysis of left atrial appendage closure (LAAC) has not yet been investigated.
WHAT DOES THIS STUDY ADD?Our results suggest that LAAC has similar risks in patients with a diagnosis of ATTR-CA compared with those without a known diagnosis of the disease and might not be futile given similar 2-year survival. Although patients with a diagnosis of ATTR-CA have much higher mortality at 5 years of follow-up, it was unrelated to bleeding complications or stroke, suggesting the efficacy of LAAC. Further research is required for other subtypes of amyloidosis affecting the heart. In addition, a systematic search of ATTR-CA in candidates for LAAC might help to elucidate the prognostic impact of the disease and the particular outcomes of LAAC in this setting.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.08.001