Cardiac amyloidosis (CA) is an infiltrative disease characterized by the extracellular deposit of Congo red material.1 Of the 2 main forms of CA, diagnosis of transthyretin cardiac amyloidosis (TTRCA) has been increasing in recent years thanks to the development of imaging techniques and the option to establish a diagnosis noninvasively, avoiding the need for biopsy in most cases. Currently-available data, showing a prevalence of 13% in heart failure with preserved ejection fraction and 16% of aortic stenosis (AS) in older patients,1 have catapulted TTRCA to the leading form of CA at present, and estimations point to an even greater increase in the coming years, given the epidemiology of the disease and the aging population.2
Fortunately, the outlook for patients with TTRCA is changing.3,4 Until recently, the diagnosis in these patients involved substantial delay, and cases were identified in advanced stages. Along with the lack of a specific treatment, this led to a bleak prognosis.5,6 Now, patients are diagnosed earlier, and the development of specific treatments that change the natural history of the disease heralds an improved survival.3 This new situation offers hope to patients and their families, at the same time presenting further challenges to clinicians.
Although in most patients TTRCA manifests mainly as heart failure,1 the management of conduction defects and atrial arrhythmias such as atrial fibrillation (AF) is an important aspect of the care of these patients. In particular, the prevalence of AF is very high in the nonhereditary form of TTRCA (previously known as the senile form), being found in up to 52.5% of patients at first assessment.7
Embolic events have been reported as a common complication in patients with TTRCA and AF, affecting up to 16% of patients.7 Furthermore, it appears that even patients with TTRCA without AF have an increased embolic risk.7
Regarding thromboembolic prevention, multiple questions remain to be resolved. There are few data on the incidence and prevalence of embolic events in TTRCA. In addition, until recently, the data were from combined series with patients with primary or light chain (AL) CA. Also, the performance of the CHA2DS2-VASc score in the prediction of embolic events in TTRCA with or without AF is unknown, and should therefore not be used for thromboembolic risk assessment in this population.3,7 Furthermore, patients with CA are known to have a tendency to bleeding due to vessel wall fragility secondary to amyloid deposits. However, there is also no validated hemorrhagic risk estimation for use in TTRCA.8 Recent data indicate a high incidence of major bleeding with clinical consequences in patients with TTRCA, regardless of whether they are anticoagulated, underscoring the difficulty in evaluating the risk-benefit balance in this condition.7 Lastly, the new oral anticoagulants appear to be as safe as vitamin K antagonists and there have been no differences found in the rate of embolism between the 2 strategies, although labile INR is associated with a higher rate of embolism.7,9
Until recently, patients with CA were denied invasive procedures and new techniques due to the apparent poor prognosis conferred by the myocardial disease. However, with the new specific treatments available for TTRCA and others on the horizon, it becomes necessary to know the outcomes of other therapeutic options besides classic supportive treatment.
In the recent article by Amat-Santos et al. published in Revista Española de Cardiología, the authors present the clinical and safety outcomes of left atrial appendage closure in patients with TTRCA.10 With this objective in mind, they analyzed data from a multicenter, retrospective study that included 1159 patients from 11 national centers and compared the results from 40 patients with a definitive diagnosis of TTRCA with the rest of the cohort (control group). The proportion of patients with TTRCA increased from 2015, possibly reflective of the increased diagnosis thanks to the emergence of nuclear medicine techniques. Among patients with TTRCA, the main indication for left atrial appendage closure was previous systemic bleeding (77.5%), followed by embolism/stroke despite oral anticoagulation (12.5%), and high bleeding risk (10%); this was similar in the remaining patients. According to the authors, there were also no significant differences in terms of the type of device used and the outcomes obtained.
In the cohort, left atrial appendage thrombus was detected before starting the procedure in 7.5% of patients with TTRCA, versus 2.9% of the control group, in line with previous studies such as that by the Mayo Clinic, in which they reported a high proportion of left atrial appendage thrombus in patients with CA, detected on transesophageal echocardiography before electrical cardioversion.11
The authors demonstrate that the procedure was successful in all the patients with TTRCA despite these patients being older, with more comorbidities and higher hemorrhagic and thrombotic risk.10 Not unsurprisingly, the patients with TTRCA had a significantly higher rate of transient ischemic attack prior to the procedure and more major bleeding events than the control group. Undoubtedly, all these characteristics reflect a more vulnerable population; despite this, the rate of periprocedural acute complications was very low (5% during the hospital stay) and comparable to the control group, with no in-hospital mortality.
Regarding the results from long-term follow-up, ischemic and hemorrhagic stroke were more common in patients with TTRCA (2.5% vs 5% and 0.8% vs 2.5%, respectively) although differences were not statistically significant, probably due to insufficient statistical power. When these events were combined, the differences in terms of the rate of events at 2 years did become significant (3.5% vs 10.8%; P=.048). Long-term, the 5-year mortality was significantly higher among patients with TTRCA but according to the authors it was not related to hemorrhagic or embolic events (40% vs 19.2%; P=.001).
At present, the indication for left atrial appendage closure in patients with AF remains unclear. The current guidelines state that left atrial appendage closure may be considered for the prevention of stroke in patients with AF and contraindication for long-term anticoagulation (IIb B recommendation).12 However, left atrial appendage closure may also be considered for patients with high bleeding risk, as it has demonstrated noninferiority to the new oral anticoagulants in recent studies.13,14 This strategy also seems reasonable for patients with embolic events despite appropriate anticoagulation, although the evidence is limited.14
The results of the study by Amat-Santos et al.10 are of great interested due to their novelty, considering there is no evidence in the literature on the outcomes and safety of left atrial appendage closure in patients with TTRCA. One of the study's strengths is its multicenter design. However, it is limited by its retrospective, observational nature and the number of patients included with TTRCA, meaning a statistical power too low to draw more solid conclusions. Furthermore, they did not systematically exclude the presence of TTRCA in the control group. In addition, most of the patients with TTRCA had an initial stage of the disease (National Amyloidosis Centre stage 1), making extrapolation of the results more difficult and raising the question of whether the procedure would be useful in all patients with TTRCA. Also, 22.5% of the patients with TTRCA did not have a genetic study, with little representation of the hereditary forms in this cohort, so the combined analysis of the 2 forms could constitute a confounding factor as these are 2 distinct diseases.
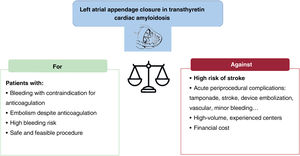
In summary, the data shown provide evidence on the safety and feasibility of left atrial appendage closure in patients with TTRCA, accepting a low rate of nonfatal complications, but with a substantial rate of combined stroke at 2 years: > 10%. Based on bleeding risk and comorbidities, left atrial appendage closure in patients with TTRCA may be an alternative in select patients with contraindication for anticoagulation or with atrial thrombus despite anticoagulation, after assessing other options (change of anticoagulant, increased target INR, or addition of an antiplatelet agent if there is persistent atrial thrombus) and a thorough evaluation that weighs up the risks and benefits (figure 1). The high risk of postprocedural stroke observed in this cohort should be borne in mind and the complications associated with the procedure should not be underestimated. In the long-term, factor XIa inhibitors, which appear to confer a minor bleeding risk, could also tip the balance toward oral anticoagulation.15
The future care of chronic patients with TTRCA represents a clinical challenge in this population with special medical needs and limited scientific evidence. More large, prospective studies are needed to throw light on this challenging but interesting area.
FUNDINGThis study was performed in part thanks to grants from the Instituto de Salud Carlos III (JR18/00038, PI18/0765 and CM20/00101).
CONFLICTS OF INTERESTE. Gonzalez-Lopez has participated as a speaker in activities organized by Pfizer, Alnylam and Eidos and has received consultancy honoraria from Pfizer, Proclara, and Akcea. F. de Frutos declares speaker's honoraria from Pfizer. E. Gonzalez-Lopez and F. de Frutos declare that Pfizer, Alnylam, and Eidos have funded training projects and research projects in their institution.