The beginning of the year saw the publication of new recommendations on echocardiographic quantification, proposing interesting changes regarding normal cutoff values, volume quantification, recommendations on 3-dimensional echocardiography, and deformation parameters.1 Other published documents included recommendations on the assessment of patients with cancer2 and acute patients3 as well as recommendations on echocardiography in hypertension.4
The Spanish Society of Cardiology, through their Department of Cardiac Imaging, issued a recommendation document on the appropriate use of handheld echocardiography devices5 (Table).
Indications for the Use of Hand-held Equipment
| 1. | Complementary to physical examination in the coronary care unit or intensive care unit |
| 2. | Initial screening in the emergency department |
| 3. | Cardiac consultation, outpatient or inpatient |
| 4. | Initial assessment in ambulance |
| 5. | Population screening programs |
| 6. | Selection of candidates for complete echocardiographic examination |
| 7. | Undergraduate and specialty training |
| 8. | Semi-quantitative evaluation of extravascular pulmonary fluid |
Reproduced with permission from Barba et al.5
Technological developments included several studies that presented the possibility of obtaining models of various diseases—particularly congenital diseases—using 3-dimensional printing, with data from 3-dimensional echocardiography or other techniques.6,7 There were also advances is the fusion of echocardiographic and fluoroscopic imaging in the areas of coronary angiography8 and interventional techniques.9
Myocardial Perfusion With Contrast and Stress EchocardiographyFrom an experimental point of view, there was continued discussion on the possibility of marking cells with contrast agents10 and of using microbubbles for therapeutic purposes, incorporating drugs that can be released and activate therapeutic targets.11
Despite the emergence of new contrasts12 and new evidence on their advantages,13 perfusion techniques have not yet been incorporated as an everyday echocardiography tool.
Stress echocardiography further increased its scope of activity beyond ischemic heart disease: use of this technique is encouraged in heart failure with preserved ejection fraction14 and in valvular heart disease.15 There was also greater interest in the study of diastolic function reference values in stress echocardiography.16
Echocardiographic calcium score after stress echocardiography was demonstrated to have a predictive value for events during follow-up.17 New analyses recommend not screening for ischemic heart disease with ischemia detection tests, including stress echocardiography, in asymptomatic low-risk patients.18
Three-dimensional EchocardiographyOne of the most interesting developments of 2015 was the publication of the updated recommendations on the use of transesophageal echocardiography. There was particular emphasis on the usefulness of this technique as a support to structural interventions, with significant reference to 3-dimensional transesophageal echocardiography.19 Furthermore, 3-dimensional echocardiography was established as advantageous and as the gold standard for the study of various aspects of cardiac anatomy and function: in one study,20 3-dimensional echo was able to determine the dynamics of the calcified mitral annulus.
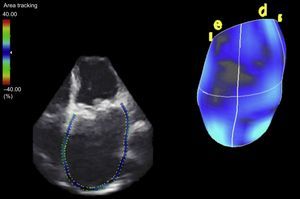
Regarding advances in myocardial deformation assessment, the studies of note were those published on the usefulness of 3-dimensional data sets; this technique received as much support as criticism.21,22 One interesting study showed the potential of new parameters obtained from 3-dimensional echocardiography, in the form of propagation imaging of left ventricular activation.23 It was also a productive year in the area of atrial mechanics assessment. The possibility of analyzing not only ventricular myocardial deformation but also atrial myocardial deformation24 was strengthened, and atrial volume is known to be an excellent prognostic marker in various heart diseases25 (Figure 1).
InterventionsOne of the previously little-explored fields in the role of imaging in structural interventions was percutaneous closure of interventricular septal defects. A notable study showed 3-dimensional echocardiography to be a highly useful tool in this procedure.26 Regarding percutaneous closure of the atrial appendage, an excellent review article makes recommended reading, detailing echocardiographic assessment and the clinical implications of the findings regarding the left atrial appendage.27 One article outlined a new proposal to measure the dimensions of the left atrial appendage using transesophageal echocardiography.28 Another article described the usefulness of complementary information obtained from cardiac computed tomography (CT) and echocardiography.29 Regarding percutaneous closure of periprosthetic dehiscence, a noteworthy article addressed the subject of communication between interventionalists and echocardiographers in this type of procedure.30
Valvular Heart DiseaseA subject attracting much attention in the field of valvular disease was quantification of aortic stenosis (AS) in patients with a paradoxical low gradient aortic stenosis (PLGAS). One of the limitations in calculating the aortic valve area by echocardiography is quantifying the area of the left ventricular outflow tract. The Leiden group contributed an original study in which they combined information from echocardiography and CT, in a population of 191 patients with severe AS on echocardiography. These authors quantified the aortic valve area by combining information from Doppler with measurement of the left ventricular outflow tract on CT. With this combination of techniques, 52% of patients with a diagnosis of severe AS with normal flow and low gradient and 12% of patients with severe AS with low flow and low gradient were reclassified as having moderate AS.31 Paradoxical low gradient aortic stenosis was established as a distinct clinical entity. The Pellika group retrospectively explored the clinical course of patients with PLGAS. The study included 1203 patients with an aortic valve area < 1cm2 and ejection fraction > 50%, of whom 78 patients met the criteria for PLGAS, and only 5% had had a previous study with severe AS and high gradient.32 The authors concluded that PLGAS is not necessarily the final stage of AS, but is an entity with specific myocardial and hemodynamic changes. Although the patients with PLGAS had a preserved ejection fraction, they had intrinsic systolic dysfunction, as demonstrated on measurement of global longitudinal strain, which has been shown to affect both prognosis33,34 and functional capacity.35 Furthermore, following transcatheter aortic valve implantation (TAVI) in patients with PLGAS, transprosthetic flow was found to remain low despite the increase in the effective valve area, as a consequence of permanently elevated systemic vascular resistance.36 The complex relationship between the progressive decrease in aortic valve area and the myocardial and vascular changes can lead to a low stroke volume. The stroke volume index was confirmed as an echocardiographic measurement with strong prognostic value across a range of values.37
Three-dimensional techniques have shown usefulness in the assessment of candidate patients for TAVI. Due to the development of new quantification tools, it is now possible to perform automatic reproducible measurements of the aortic annulus throughout the cardiac cycle.38 The superiority of echocardiography over CT in the selection of candidates for direct TAVI was also demonstrated.39 New prostheses have reduced the incidence of peri-TAVI aortic regurgitation; however, quantification of this regurgitation still has prognostic implications. As in native aortic regurgitation, cardiac magnetic resonance (CMR) was shown to be a useful technique in these patients.40,41
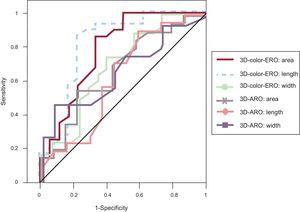
The development of 3-dimensional transesophageal echocardiography has improved anatomical assessment of the mitral valve42 and allows quantification and understanding of mitral valve apparatus remodeling in patients with functional mitral regurgitation.43 Three-dimensional transesophageal echocardiography was also established as a key part in the selection of candidates for complex percutaneous treatments, such as closure of periprosthetic dehiscence. The best parameter for predicting the degree of regurgitation and selecting the closure device was the effective regurgitant orifice area quantified with 3-dimensional transesophageal color echocardiography.44 (Figure 2).
Receiver operating characteristic curves of measurements of effective regurgitant orifice and anatomical regurgitant orifice with 3-dimensional color echography. Effective regurgitant orifice was the best predictor for correct diagnosis of moderate or severe paravalvular regurgitation. 3D-ARO, anatomical regurgitant orifice measured on 3-dimensional echocardiography with color Doppler; 3D-color-ERO, effective regurgitant orifice measured on 3-dimensional echocardiography with color Doppler. Reproduced with permission from Franco et al.4
One article that stood out for its attention to the management of aortic disease was the joint document of the American Society of Echocardiography and the European Association of Cardiovascular Imaging on the multimodal diagnostic approach, integrating the information provided by each technique. It should undoubtedly be a reference document for any cardiologist interested in this type of disease.45
Ventricular FunctionTechnological advances have made it necessary to revise the normal parameters of systolic and diastolic ventricular function46 and reevaluate the growing role of deformation techniques in various clinical scenarios.47,48 Until now, differences between devices have prevented these techniques from becoming widespread. This year, a consensus document of the European Association of Cardiovascular Imaging/the American Society of Echocardiography/Industry49 was published to standardize 2-dimensional strain quantification, thus allowing its increased clinical use.50
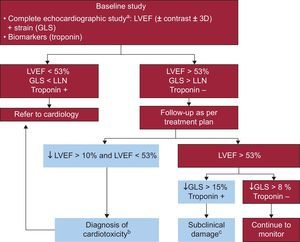
CardiomyopathiesEchocardiography is a key tool in the assessment of athletes,51 as well as in the management of cardiomyopathies.52 In patients with hypertrophic cardiomyopathy, abnormalities in segmental contractility during exercise add prognostic value to the baseline study.53 Quantification of ventricular deformation also adds prognostic value54 and helps in the differential diagnosis of cardiac disease in infiltrative diseases.55 Another important area of growing interest was cardio-oncology. Imaging techniques are an essential tool in the diagnosis of cardiotoxicity and in the monitoring of oncological treatment (Figure 3).2 The developments in this area were the registries that contain a large number of patients with long-term follow-up, which continue to clarify doubts raised by smaller studies (such as the 9% incidence of ventricular dysfunction after the use of anthracyclines at 1 year).56 Monitoring of global longitudinal strain throughout chemotherapy was shown to allow the early diagnosis of subclinical myocardial damage.57 One study showed that 1 of every 3 cancer survivors had an abnormal global longitudinal strain,58 and only early treatment of these patients with beta-blockers and angiotensin-converting enzyme inhibitors improved the chance of recovery.59
Flowchart from the guidelines of the American Society of Echocardiography/European Association of Cardiovascular Imaging on monitoring of adult patients with cancer. 3D, 3-dimensional echocardiography; GLS, global longitudinal strain; LLN, lower limit of normality according to equipment used; LVEF, left ventricular ejection fraction. These parameters identify patients at risk of ventricular dysfunction. aEchocardiographic study according to the clinical practice guidelines of the American Society of Echocardiography/European Association of Cardiovascular Imaging. bStarting treatment with angiotensin-converting enzyme inhibitors/beta-blockers is recommended. cThere are few data that support starting beta-blockers and angiotensin-converting enzyme inhibitors to prevent ventricular remodeling. Adapted with permission from Plana et al.2.
Optimal use of imaging techniques is essential in the diagnosis, treatment planning, and follow-up of patients with heart failure.60 Interesting studies were published on the role of deformation techniques in risk stratification of patients with heart failure and left ventricular ejection fraction > 50%.61,62 It seems that these techniques will provide a new opportunity to study ventricular asynchrony with echocardiography.63
CARDIAC MAGNETIC RESONANCE IMAGINGCardiac magnetic resonance is the technique of choice for assessment after acute myocardial infarction. In the acute phase of the infarct, the time elapsed between administering the contrast and acquiring the late enhancement sequences is crucial in quantifying the necrotic mass. During this phase, gadolinium can be retained in the myocardial area at risk, due to the presence of edema and inflammation, and can have slower clearance kinetics than the remote myocardium. Therefore, it is recommended to wait 20 min to 25min between administering contrast and acquiring viability sequences.64 This point is critical, as myocardial necrosis determination is used as a substitute variable in studies evaluating reperfusion strategies and as a prognostic variable of adverse events during follow-up. One randomized double-blind study demonstrated that the administration of intracoronary adenosine did not significantly reduce the size of necrosis compared with placebo.65 The most significant predictors of major cardiovascular events were reduced systolic function (< 47%), extensive necrosis (> 19% of myocardial mass), and microvascular obstruction.66,67
Stress CMR was established as an excellent technique for the diagnosis of ischemia. A meta-analysis that evaluated different noninvasive techniques in the diagnosis of significant coronary disease (by coronary angiography and fractional flow reserve) demonstrated that CMR, positron emission tomography (PET) and CT had higher sensitivity than single photon emission CT (SPECT) or stress echocardiography.68
Another field of interest in CMR was the assessment of cardiomyopathy. Chan et al69 demonstrated, in 1293 patients followed-up for 3.3 years, that myocardial fibrosis of > 15% of the myocardial mass doubled the risk of sudden death compared with that in patients without fibrosis. In patients with myocarditis, T2 mapping sequences showed higher sensitivity and positive predictive value than biopsy in the diagnosis of active myocarditis.70
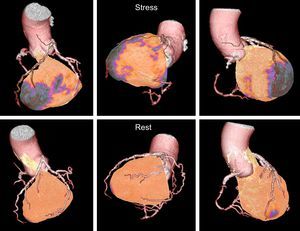
COMPUTED TOMOGRAPHYComputed tomography was confirmed as an excellent option for the diagnosis of coronary artery disease in patients with an intermediate probability (20%-90%) of ischemic heart disease. The EVINCI study compared CT, SPECT/PET, CMR, and stress echography ± fractional flow reserve in 30% to 70% stenoses. The results showed that CT had a sensitivity of 91% and specificity of 92%, which were higher than those of the other investigational techniques.71 The growing technological development and the combination of stress protocols has improved diagnostic accuracy. In the CORE320 study,72 stress CT improved the accuracy of conventional CT in the diagnosis of significant coronary artery disease (Figure 4). However, stress CT has not yet replaced conventional ischemia detection techniques.
The PROMISE study73 demonstrated that, in patients with suspected coronary artery disease, assessment with CT had the same predictive value for adverse events as other functional investigations over a follow-up of 2 years. Studies such as the CAPP trial74 demonstrated that CT improved diagnostic accuracy in the assessment of symptomatic patients with suspected coronary disease. In patients with coronary artery disease who underwent CT, planned interventions were changed in 15%, the treatment regimen was changed in 25%, and the rate of events was reduced,75 compared with conventional management.
Computed tomography is an excellent technique in the assessment of the aortic annulus and vascular access of candidate patients for TAVI. Valve calcium score (> 1274 Agatston units in women and > 2065 Agatston units in men) was associated with higher mortality in patients with medically treated severe AS.76
NUCLEAR MEDICINEMyocardial PerfusionRecently, 2 large series were published, evaluating the role of myocardial perfusion studies. Romero-Farina et al77 defined the warrantee period of a normal myocardial perfusion SPECT (period of remaining low-risk [< 1% events/year] regarding need for repeat investigation during that period) in a series with 3000 normal studies, with a follow-up of 5 years. These authors concluded that this period depended on the clinical characteristics of the patient, the type of stress test performed, and the left ventricular ejection fraction. This same group analyzed differences by sex in the predictive value of stress-rest gated SPECT, in 2414 patients (1438 women).78
Multimodal ImagingPizzi et al79 evaluated the usefulness of SPECT, CT, and fusion hybrid imaging in patients with stable angina requiring invasive coronary angiography. Computed tomography was superior in the diagnosis of multivessel disease and the detection of the culprit vessel. SPECT was a functional complement to invasive coronary angiography in detecting the most ischemic territory. In patients with SPECT prior to invasive coronary angiography, SPECT-CT fusion imaging did not change therapeutic management.
The initial article on the EVINCI trial multicenter European protocol71 was published. In patients with a low prevalence of coronary disease and stable angina, studied with all types of cardiac imaging techniques, the optimal diagnostic technique for evaluation of coronary artery disease was combined coronary CT and perfusion SPECT.
Quantification of Regional Blood Flow and Coronary ReserveAbsolute quantification studies of regional myocardial blood flow performed with PET acquired new relevance.80,81 A normal quantitative value of coronary reserve practically excludes severe coronary artery disease (high negative predictive value), and quantitative evaluation of coronary reserve has a prognostic value independent of coronary anatomy, as it provides information on diffuse atherosclerosis and microvascular disease.
One of the first studies on quantification of coronary reserve using SPECT/CT offered promising data for the use of this technique in the near future.82
AmyloidosisThere was new information in the field of cardiac amyloidosis.83 With the availability of the classic 99mTc-pyrophosphates (which are positive in the transthyretin variants of amyloidosis) and the commercialization of various radiopharmaceuticals as PET markers specific for amyloid deposits (such as 18F-florbetapir), images can be obtained of amyloid light chains in the myocardium.
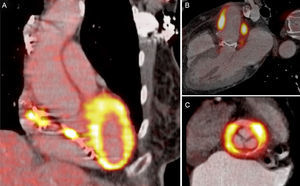
Positron Emission Tomography and Computed Tomography in EndocarditisTwo studies were helpful regarding the diagnosis of infection of implantable devices (pacemakers and implantable cardioverter-defibrillators) using PET/CT with 18F-fluorodeoxyglucose: they describe a very high diagnostic accuracy,84 and the ability to demarcate the extent of infection.85
The publication by Pizzi et al86 involved a series of 92 patients with suspected prosthetic or device endocarditis investigated using gated PET with 18F-fluorodeoxyglucose combined with cardiac CT angiography. These authors found that this technique made patient classification faster and easier, because it reduced the percentage of cases defined as possible endocarditis from 55% to 5%, with 95% of patients correctly classified. Sensitivity was 91% and specificity was 90.6%, being somewhat better for implantable devices (87.5% and 100.0%) than for prosthetic valves (87.2% and 92.0%). The PET/CT technique was included in the guidelines of the European Society of Cardiology as a new major criterion for endocarditis, based on its high negative predictive value in patients with prostheses beyond the first 3 months after implant87 (Figure 5).
CONFLICTS OF INTERESTNone declared.