Developments in cancer therapeutics such as immune checkpoint inhibitors (ICIs) have improved outcomes but have also been associated with cardiovascular complications. A variety of mechanisms responsible for cardiovascular damage have been proposed, including acute coronary syndromes, unmasking occult underlying cardiovascular disease, arrhythmias, myocarditis, and pericarditis as part of a systemic immune syndrome as a consequence of ICI treatment. Furthermore, as the clinical use of ICI therapy is increasing rapidly, concern is growing about the long-term sequelae in survivors.
Cardiac magnetic resonance (CMR) is useful to provide a diagnosis in those patients with suspected myocardial injury. To date, all studies in ICI cardiotoxicity have explored the ability of CMR to detect myocarditis exclusively.1 The aim of this study was to describe the presence, type and extent of myocardial injury in a well-defined cohort of oncological patients receiving treatment with ICIs.
This cross-sectional, observational, cohort study consecutively recruited patients scheduled for therapy with ICIs between April, 2019 and October, 2020 at the University Hospital of Salamanca. The study protocol was approved by the University Hospital of Salamanca ethics committee and participants provided written informed consent. Patients underwent a 1.5-Tesla CMR (Philips Healthcare, Netherlands) including cardiac morphology and function assessment, T2-weighted short-tau triple inversion-recovery (T2W-STIR) sequence, late gadolinium enhancement (LGE), T1 modified Look Locker imaging (T1-MOLLI-with-5(3)3 acquisition scheme) before and 15minutes after gadolinium, and T2 (multiecho gradient-spin-echo sequence) mapping according to recommendations.2 Mapping postprocessing was performed using the Medis 2.1 software, plotting the region-of-interest in the left ventricle midseptum segment in short axis. CMR diagnosis of active myocarditis was based on the published Lake Louise expert recommendations3; the main criteria in this document are a positive edema-sensitive T2-based marker (T2-weighted images or T2 mapping) and positive T1-based tissue characterization markers (abnormal T1 relaxation time or extracellular volume or LGE).3 Because myocarditis was diagnosed according to these T2- and T1-based CMR markers, we used as controls CMR imaging from 50 sex- and age-matched individuals without cardiac disease from a local population-based sample (NCT03429452).4
Categorical variables are described as percentages and continuous variables as median (interquartile range [IQR]). The Fisher exact test was used to compare proportions across groups. Nonparametric tests at the ordinal level were used for independent (Mann-Whitney U-test) samples. Analyses were performed using SPSS Statistics, version 22 (IBM, Armonk, United States).
A total of 53 consecutive patients were enrolled. The median age was 65 [56-71] years, 85% of patients were male, 72% had cardiovascular risk factors, 17% had a history of cardiovascular disease, and 60% were taking concomitant cardiovascular medications. Before treatment with ICI, 11% patients had surgery, 57% received chemotherapy, and 26% thoracic radiation (table 1). The median time since the beginning of ICI was 222 [19-1033] days, with the median number of cycles received being 13 [6.5-23.5]. CMR identified some degree of myocardial injury in 35 (66%) patients (table 2). Decreased left ventricular ejection fraction (LVEF) was present in 10 (19%) patients (8 with LVEF between 35% to 55% and 2 with LVEF <35%), with 3 cases due to myocardial infarction, 2 cases due to aortic regurgitation, and 5 cases without LGE. In total, LGE was present in 11 (21%) patients, with subendocardial or transmural LGE in 6 (11%), subepicardial LGE in 2 (4%), and midwall LGE in 3 (6%) patients. Active myocarditis, fulfilling Lake Louise criteria, was observed in 7 (13%) patients. In patients with myocarditis, compared with those without, values of T2 mapping (65ms [63-77] vs 55ms [51-59]); P=.005) were significantly higher, with no differences between both groups on native T1 mapping (1086ms [1012-1125] vs 998ms [959-1032]; P=.081) or extracellular volume (34% [29-37] vs 26% [25-28]; P=.159). LGE was identified in 3 (43%) of 7 patients with myocarditis criteria and in 8 of 46 (17%) without (P=.075). In addition, we found no differences in the presence of regional wall motion abnormalities (14% vs 15%; P=.937) between patients with and without myocarditis-like criteria. Finally, we observed mild pericardial effusion in 4 (7%) patients, all without myocarditis. No other complications were detected.
Clinical characteristics
| All participants(N=53) | No myocardial injury(n=18) | Some degree of myocardial injury(n=35) | P | |
|---|---|---|---|---|
| Age at start ICI, y | 65 [56-71] | 64 [54-70] | 67 [59-73] | .358 |
| Male sex | 45 (85) | 16 (89) | 29 (83) | .701 |
| Cardiovascular risk factors | ||||
| Hypertension | 15 (28) | 6 (33) | 9 (26) | .834 |
| Diabetes mellitus | 7 (13) | 1 (6) | 6 (17) | .401 |
| Dyslipidaemia | 25 (47) | 7 (39) | 18 (51) | .562 |
| Smoker | 7 (13) | 5 (28) | 2 (6) | .037 |
| COPD | 13 (25) | 5 (28) | 8 (23) | .743 |
| Prior coronary artery disease | 3 (6) | 1 (6) | 2 (6) | .999 |
| Prior stroke | 1 (2) | 1 (6) | 1 (3) | .340 |
| Prior pulmonary embolism | 2 (4) | 1 (6) | 1 (3) | .416 |
| Prior peripheral artery disease | 4 (8) | 1 (6) | 3 (9) | .999 |
| Prior atrial fibrillation | 2 (4) | 0 | 2 (6) | .799 |
| Cardiological treatment prior ICI | ||||
| Beta-blocker | 8 (15) | 1 (6) | 7 (20) | .240 |
| ACE inhibitor or ARB | 12 (23) | 5 (28) | 7 (20) | .730 |
| Calcium antagonist | 4 (7) | 1 (6) | 3 (9) | .999 |
| Statin | 21 (40) | 7 (39) | 14 (40) | .999 |
| Aspirin or/and ticagrelor | 8 (15) | 2 (11) | 6 (17) | .864 |
| Anticoagulants | 5 (10) | 2 (11) | 3 (9) | .212 |
| Antidiabetics | 7 (13) | 1 (6) | 6 (17) | .126 |
| Primary cancer type | .272 | |||
| Nonsmall lung cancer | ||||
| Squamous | 12 (23) | 3 (17) | 9 (26) | |
| Nonsquamous | 18 (34) | 8 (44) | 10 (29) | |
| Not otherwise specified | 9 (17) | 4 (22) | 5 (14) | |
| Renal cell cancer | 6 (11) | 0 | 6 (17) | |
| Melanoma | 4 (7) | 3 (17) | 1 (3) | |
| Urothelial cancer | 2 (4) | 0 | 2 (6) | |
| Head and neck cancer | 1 (2) | 0 | 1 (3) | |
| Endometrial cancer | 1 (2) | 0 | 1 (3) | |
| Prior treatment to ICI | ||||
| Surgery | 6 (11) | 3 (17) | 3 (9) | .378 |
| Chemotherapya | 30 (57) | 9 (50) | 21 (60) | .487 |
| Cisplatin | 25 (47) | 8 (44) | 17 (49) | .776 |
| Pemetrexed | 7 (13) | 4 (22) | 3 (9) | .165 |
| 5FU/capecitabine | 3 (6) | 1 (6) | 2 (6) | .981 |
| Gemcitabin | 3 (6) | 0 | 3 (9) | .201 |
| Taxane | 17 (32) | 5 (28) | 12 (34) | .631 |
| Vinca alcaloids | 5 (9) | 1 (6) | 4 (11) | .488 |
| Somatulin | 1 (2) | 0 | 1 (3) | .469 |
| Etoposide | 1 (2) | 0 | 1 (3) | .469 |
| Tyrosine kinase inhibitorsb | 6 (11) | 1 (6) | 5 (14) | .464 |
| Immunotherapyc | 1 (2) | 1 (6) | 0 | .291 |
| Thoracic radiation | 14 (26) | 6 (33) | 8 (23) | .705 |
| Immunotherapy regimen | .765 | |||
| Anti-PD1 | 48 (91) | 16 (89) | 32 (91) | |
| Anti-PDL1 | 5 (9) | 2 (11) | 3 (9) | |
| Laboratory measures | ||||
| Troponin T, pg/mL | 1010 [624-1696] | 834 [522-1238] | 1071 [726-1713] | .125 |
| NT-proBNP, pg/mL | 186 [70-510] | 113 [62-415] | 197 [84-719] | .512 |
| Glomerular filtration rate, mL/min x 1.73 m2 | 79 [60-89] | 87 [74-90] | 77 [58-85] | .046 |
5FU, 5 fluorouracil; ACE, angiotensin-converting enzyme; AMI, acute myocardial infarction; ARB, angiotensin II receptor blockers; COPD, chronic obstructive pulmonary disease; ICI, immune checkpoint inhibitor; NT-proBNP, N-terminal probrain natriuretic peptide.
Values are expressed as No. (%) or median [interquartile range].
Chemotherapy agents consisted of, in decreasing frequency: carboplatin, pemetrexed, etoposide, cisplatin, capecitabine, 5FU, gemcitabine, somatuline and vinca alcaloids.
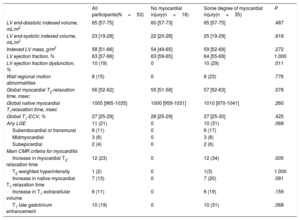
CMR features
| All participants(N=53) | No myocardial injury(n=18) | Some degree of myocardial injury(n=35) | P | |
|---|---|---|---|---|
| LV end-diastolic indexed volume, mL/m2 | 65 [57-75] | 60 [57-73] | 65 [57-75] | .487 |
| LV end-systolic indexed volume, mL/m2 | 23 [19-28] | 22 [20-28] | 25 [19-29] | .618 |
| Indexed LV mass, g/m2 | 58 [51-66] | 54 [49-65] | 59 [52-69] | .272 |
| LV ejection fraction, % | 63 [57-66] | 63 [59-65] | 64 [55-69] | 1.000 |
| LV ejection fraction dysfunction, % | 10 (19) | 0 | 10 (29) | .011 |
| Wall regional motion abnormalities | 8 (15) | 0 | 8 (23) | .776 |
| Global myocardial T2-relaxation time, msec | 56 [52-62] | 55 [51-58] | 57 [52-63] | .078 |
| Global native myocardial T1relaxation time, msec | 1005 [965-1035] | 1000 [959-1031] | 1010 [970-1041] | .260 |
| Global T1-ECV, % | 27 [25-29] | 26 [25-29] | 27 [25-30] | .425 |
| Any LGE | 11 (21) | 0 | 10 (31) | .068 |
| Subendocardial or transmural | 6 (11) | 0 | 6 (17) | |
| Midmyocardial | 3 (6) | 0 | 3 (8) | |
| Subepicardial | 2 (4) | 0 | 2 (6) | |
| Main CMR criteria for myocarditis | ||||
| Increase in myocardial T2-relaxation time | 12 (23) | 0 | 12 (34) | .005 |
| T2-weighted hyperintensity | 1 (2) | 0 | 1(3) | 1.000 |
| Increase in native myocardial T1-relaxation time | 7 (13) | 0 | 7 (20) | .081 |
| Increase in T1-extracellular volume | 6 (11) | 0 | 6 (19) | .159 |
| T1-late gadolinium enhancement | 10 (19) | 0 | 10 (31) | .068 |
CMR, cardiac magnetic resonance; LV, left ventricular; ECV, extracellular volume.
Values are presented as No. (%) or median [interquartile range].
Myocardial injury is common in patients receiving ICI treatment and is not exclusively due to the development of active myocarditis. CMR can frequently reveal occult coronary artery disease, myocarditis-like pathology or other myocardial injury due to (as in our cohort) valvulopathy.5 In this single-center experience, myocardial injury corresponding to myocarditis-like pathology was observed in 13% of the CMR studies with an additional 11% of patients showing CMR findings consistent with ischemic etiology. The finding of unknown valvular heart disease such as aortic regurgitation could be due to the previous radiotherapy received6 or could be a casual finding present before the start of ICI. Since CMR is the noninvasive modality most suited to identify the presence, type, and extent of myocardial injury, it should be used to monitor patients receiving ICI treatment to detect pathology occurring before the appearance of regional wall motion abnormalities or LV dysfunction. A longitudinal design, instead of the current cross-sectional design, would be preferable in future studies, in which CMR information before the start of immunotherapy will better establish sequences of cardiac injury.
FUNDINGThis study was supported by the Spanish Cardiovascular Network (CIBERCV), by a Project of Excellence (PIE14/00066) and by a Research Project (PI17/00145); all national, public and competitive resources from the Instituto de Salud Carlos III (Ministerio de Ciencia e Innovación, Spain) founded by the EU–European Regional Development Fund.
AUTHORS’ CONTRIBUTIONSB. Barrio-Collado, A. Martin-Garcia, P.L. Sanchez and Juan Jesús Cruz conceived and designed the study; R. Eiros and A. Martin-Garcia contributed to the literature search; B. Barrio-Collado, A. Martin-Garcia, R. Eiros and C. Sanchez-Pablo contributed by clinically evaluating the participants; B. Barrio-Collado and R. Eiros performed clinical data collection; R. Eiros and P.L. Sanchez contributed to data analysis and interpretation; R. Eiros and P.L. Sanchez contributed to the writing of the report.
CONFLICTS OF INTERESTThe authors have no conflicts of interest.