We studied 21 patients who presented with a diagnosis of pentalogy of Cantrell. Their mean age was 40 days. All patients presented with congenital heart disease. Six presented with ectopia cordis. Every patient was subjected to echocardiography. Double outlet right ventricle, an atrial septal defect and dextrocardia were seen in 5 patients (24%). Four patients survived. Seventeen died, 12 from sepsis or septic shock. Autopsies were performed on 10 patients. All of the ectopia cordis patients died. Pentalogy of Cantrell is uncommon, and its association with ectopia cordis indicates poor prognosis.
Keywords
The literature contains few reports of ectopia cordis (EC). Its incidence is just 5.5-7.9/million births and it represents just 0.5%-0.8% of congenital heart disease.1, 2, 3, 4, 5
The first case was reported by Neil Stenonis, who described a child with an open sternal line and protruding heart, liver and spleen.2 In 1706, Haller and Martínez separately reported cases of EC.6, 7 The etiology of this problem is probably sporadic and multicausal transmission; the pathogeny of the problem is unknown.8
In 1958 Cantrell, Haller and Ravitch associated EC with other anomalies of the mid-line, including supraumbilical defects and defects of the lower sternum, the anterior diaphragm, the pericardium and intracardium, which together came to be known as pentalogy of Cantrell (PC).9 Cardiological diagnosis can be made via fetal or postnatal transthoracic ultrasound.3 The present work reports the prevalence of heart disease and the clinical progress of a series of patients cared for at our center.
MethodsA retrospective, longitudinal, observational study was made of the medical records of patients with PC who received assistance between February 1971 and January 2009. Initial diagnoses were made using M mode or two-dimensional transthoracic echocardiography employing an Aloka Model 870 apparatus. After the year 2000, M mode, two-dimensional, continuous Doppler, pulsed Doppler or color Doppler echocardiography was performed using a Hewlett Packard 5.500 apparatus with 5-8MHz transducers. The classification of Cantrell and Davies was used to define the site of clinical involvement.9, 10
A descriptive statistical analysis was performed, categorical variables were recorded as simple frequencies, continuous variables as measures of central tendency, and associations were sought using the χ2 test.
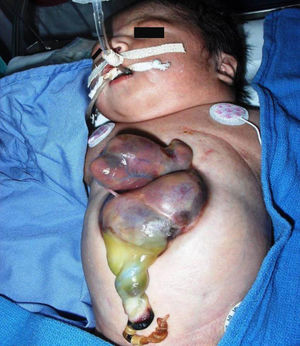
ResultsOver the 38-year study period, 21 patients were diagnosed with PC, of whom 6 suffered EC (Figure 1).
Figure 1. Ectopia cordis.
Ages at the time of diagnosis ranged from 1-365 days (mean 40 days). Locations of the PC defects were: supraumbilical (omphalocele) in 13 patients (62%), the lower sternum (bifid sternum) in 13 (62%), the lower diaphragm (diaphragmatic hernia) in 11 (52%), and the pericardium in 1 (9.5%) (Table 1).
Table 1. Clinical Characteristics of Patients With Pentalogy of Cantrell.
| Patient | Classification | Clinical problem |
| 1 | V | EC, C |
| 2 | IV, V | EC, C, AP, prosencephaly |
| 3 | V | EC, C |
| 4 | I, V | EC, C, O |
| 5 | I, V | EC, C, O |
| 6 | I, V | EC, C, O, cleft lip and palate |
| 7 | I, II, V | AX, C, O |
| 8 | II, V | AX, C, diastasis recti abdominis |
| 9 | I, II, V | G, C, cleft sternum |
| 10 | II, III, V | AX, C, DH, atrophy of the corpus callosum, athymic |
| 11 | I, II, III, V | AX, C, O, DH |
| 12 | I, II, III, V | AX, C, G, DH |
| 13 | I, II, III, V | AX, C, O, DH |
| 14 | I, II, III, V | AX, C, O, DH, absence of left pectoral muscle |
| 15 | II, III, V | AX, C, DH |
| 16 | III, V | C, DH |
| 17 | II, III, V | C, DH, xyphoid deformity, cleft lip and palate |
| 18 | I, II, V | AX, C, G, hare lip |
| 19 | I, III, V | DH, C, O |
| 20 | I, II, III, V | AX, C, DH, O |
| 21 | I, II, III, V | AX, C, DH, G |
I, supraumbilical defect; II, defect of the lower sternum; III, defect of the anterior diaphragm; IV, defect of the pericardium; O, omphalocele; V, intracardiac defect; AP, absence of pericardium; AX, absence of xyphoid; C, cardiopathy (heart disease); DH, diaphragmatic hernia; EC, ectopia cordis; G, gastroschisis.
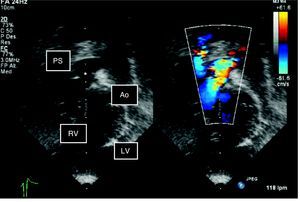
All patients were diagnosed extrauterinely (Figure 2); four underwent cardiac catheterization and 10 underwent autopsy.
Figure 2. Two-dimensional echocardiogram in color: subcostal view of the outlet tract. Note the double outlet right ventricle and pulmonary stenosis. Ao: aorta; LV: left ventricle; PS: pulmonary stenosis; RV: right ventricle.
All 21 patients suffered congenital heart disease. Nine patients (43%) suffered non-complex heart disease, 4 patients (19%) had dextrocardia (2 with an atrial septal defect [ASD], 1 with ASD and persistent ductus arteriosus [PDA], and 1 with a ventricular septal defect [VSD] with associated PDA), and 5 patients (24%) had left-right short circuits (2 with ASD, 2 with VSD, ASD and PDA, and 1 with VSD and PDA). Twelve patients (57%) presented with complex heart disease: 1 patient (5%) with dextrocardia, ASD, left ventricular diverticulum (LVD) and right isomerism; 5 patients (24%) with double outlet right ventricle (DORV) (1 with atrioventricular discordance and ASD, 1 with pulmonary stenosis [PS], 1 with PDA, and 2 with Taussig-Bing syndrome, PDA and left superior vena cava [LSVC]); 3 patients (14%) with a hypoplastic left ventricle (2 of these patients had LVD, PDA and LSVC and 1 had mitral atresia; transpositions of the great arteries were seen in 1 patient who also had VSD and PS), 1 patient had pulmonary atresia with a complete interventricular septum and LSVC; and the last patient had a single atrium and ventricle and no pericardium (Table 2).
Table 2. Heart Diseases of Patients With Pentalogy of Cantrell.
| Patient | Heart disease |
| 1 | Dextrocardia, ASD * |
| 2 | Single atrium, tricuspid stenosis, VSD, HLV, PS * |
| 3 | LVD, HLHS, ASD, PDA, LSVC * |
| 4 | Pulmonary atresia, PDA, ASD, LSVC * |
| 5 | Atrioventricular discordance, ASD, DORV * |
| 6 | ASD * |
| 7 | ASD, VSD, PDA |
| 8 | RVD, ASD |
| 9 | LVD, HLHS, LSVC |
| 10 | DORV, PS |
| 11 | VSD, PDA |
| 12 | DORV, PDA |
| 13 | Taussig-Bing syndrome type, DORV |
| 14 | Dextrocardia, ASD |
| 15 | Taussig-Bing syndrome type, DORV, PDA, LSVC |
| 16 | Dextrocardia, PDA |
| 17 | LVD, dextrocardia, right isomerism, ASD |
| 18 | HLHS with mitral atresia |
| 19 | Dextrocardia, VSD, PDA |
| 20 | Transposition of the large arteries, VSD, PS, PDA |
| 21 | VSD, ASD, PDA |
ASD, atrial septal defect; DORV, doublet outlet right ventricle; HLHS, hypoplastic left heart syndrome; HLV, hypoplastic left ventricle; LSVC, left superior vena cava; LVD, left ventricle diverticulum; PDA, persistence of the ductus arteriosus; PS, pulmonary stenosis; RVD, right ventricle diverticulum; VSD, ventricular septal defect.
* With ectopia cordis.
Of the 12 patients with complex heart disease, 4 suffered EC. Of the 9 patients who had non-complex heart disease (ASD, VSD or PDA), 2 had EC with ASD and dextrocardia and other with ASD only (χ2=0.81; P=.59). No significant association was seen between EC and either type of heart disease.
All patients received pharmacological treatment. Non-cardiac surgery to repair mid-line defects was performed on 7 patients (33%). Four patients (19%) – 1 with EC and 3 without - underwent heart surgery. None of the 4 patients (19%) who survived had EC. Seventeen patients (81%) died between the first day and fifth month of life. The causes of death were: sepsis 6 patients, septic shock 4 patients, cardiogenic shock 3 patients, heart failure 1 patient, multiorgan failure 1 patient, heart failure plus sepsis 1 patient, and mixed (cardiogenic plus septic) shock 1 patient.
All patients who presented with EC died, as did 73% of those who did not have EC. Ten (59%) of the 17 patients who died underwent autopsy; anatomopathological and clinical diagnoses were in agreement in all cases.
DiscussionThe embryonic problem associated with EC lies in the abnormal migration of the splanchnic and somatic mesoderm. This affects the development of the heart and the major vessels, with the premature breakage of the chorion or vitelline sac at around day 14-18 of gestation leading to a mid-line defect.7, 11
The literature reveals some 80% of patients with PC to have congenital heart disease. The most common problems are VSD (100%), ASD (53%), PS (33%), tetralogy of Fallot (TF) (20%) and LVD (20%).12, 13 All the present patients (with or without EC) suffered congenital heart disease, with the different forms showing different prevalence. The most common heart abnormalities were DORV (5 patients) and ASD (4 patients), although two more patients were associated with PDA and VSD. Dextrocardia was also quite commonly seen while ventricular diverticulum was less frequent (Table 2).
The patients with EC more commonly suffered complex heart disease, but not significantly so with PC also suffered associated malformations7, 12 (Table 1).
Magnetic resonance was a useful diagnostic aid when examining thoracoabdominal lesions. Echocardiography was, however, performed in all patients since it reveals intracardial malformations and hemodynamic problems.
It is commonly recommended that initial surgery treat thoracoabdominal defects when these are not restrictive, and that heart lesions be corrected later.11, 14, 15 However, other authors recommend the correction of intracardiac lesions before making any attempt to reconstruct the thorax and abdominal walls, especially if the association of lesions is complex.2, 5, 8, 16, 17 The main problem to avoid in the peri- and postoperative periods is the appearance of high abdominal and intrathoracic pressure. This reduces the venous return, inducing a reduction in cardiac output and the appearance of arrhythmias.14 Mortality in children with EC is high; no more than 5% survive.11 Children with PC who also have EC, pulmonary hypoplasia or complex heart disease, or who undergo surgery late, all face a poor prognosis.3 Very few such children survive attempts at surgical repair, the main causes of death being tachy-arrhythmias, bradycardia, low blood pressure, rupture of the diverticulum, and heart failure.14, 18 Nonetheless, some children with mild thoracoabdominal abnormalities have survived to adulthood.19
Conflicts of interestNone declared.
Received 22 April 2010
Accepted 17 August 2010
Corresponding author: Servicio de Cardiología, Avda. Insurgentes Sur 3700-C, Colonia Insurgentes Cuicuilco, Delegación Coyoacán, 04530 México DF, Mexico. derubens@hotmail.com