Long QT syndrome (LQTS) is characterized by a prolonged QT interval and ventricular arrhythmias.1 The condition has been linked to more than 17 genes (encoding potassium, sodium, and calcium channels), with 75% of those affected having a KCNQ1 (LQTS1), KCNH2 (LQTS2), or SCN5A (LQTS3) mutation. In at least 1% to 5% of cases, mutations are detected in other genes such as CACNA1C, associated with Timothy syndrome or LQTS8.1,2
We report a case of Timothy syndrome that first presented as peripartum cardiomyopathy (PPCM), without any other extracardiac manifestations, an entity recently named cardiac-only Timothy syndrome (COTS).1
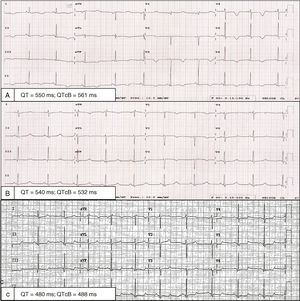
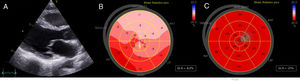
A 32-year-old woman presented with dyspnea 1 week after delivery. The patient had had another pregnancy a number of years previously, without complications. Although hemodynamically stable, she had signs of systemic and pulmonary congestion. Electrocardiography revealed generalized T-wave inversion with a Bazett-corrected QT interval (QTc) of 561ms (Figure 1A). She had not received any QT-prolonging drugs and had no electrolyte abnormalities. Transthoracic echocardiography indicated a normal-size left ventricle, without hypertrophy, with moderate ventricular dysfunction (left ventricular ejection fraction [LVEF], 36%) and general hypokinesia. The global longitudinal strain was –8.2% (Figure 2A and B, ). In-hospital telemetry found no extrasystoles or arrhythmias. After treatment with diuretics, bisoprolol, and enalapril, she progressively improved and was discharged after 7 days. However, the generalized T-wave inversion in V1 to V4 on electrocardiography persisted, as well as a QTc of 532ms (Figure 1B).
The clinical course was satisfactory; transthoracic echocardiography at 6 months revealed a normal LVEF (68%) and a global longitudinal strain of –23% (Figure 2C, ). Cardiac magnetic resonance indicated normal biventricular function and size, without delayed enhancement. The T-wave inversion was no longer visible on electrocardiography, but the QTc prolongation persisted (Figure 1C).
A genetic study was performed via massively parallel sequencing with a panel of 218 genes linked to channelopathies and cardiomyopathies. The p.Arg518Cys variant was detected in the CACNA1C gene (exon 12), which is classified as pathogenic according to current criteria.1,3,4 It can be considered a de novo mutation because the patient has no family history of sudden cardiac death or cardiomyopathy and her parents and child have a normal QTc, without structural heart disease, and are not carriers of the variant.
She is currently asymptomatic and maintains a QTc<500ms by taking beta-blockers and avoiding QT-prolonging agents.
The CACNA1C gene encodes the alpha-1 subunit of the voltage-dependent L-type calcium channel; it consists of 4 interconnected homologous domains (DI–DIV), each containing 6 transmembrane segments (S1–S6). It is essential for cell excitability, myocyte contraction, regulation of gene expression, and the plateau phase of the action potential.1 Pathogenic variants resulting in loss of channel function have been linked to J-wave syndromes, whereas gain of function variants have been associated with 4 different phenotypes1,2:
- a)
Timothy syndrome: characterized by LQTS, syndactyly, cardiac abnormalities, facial dysmorphisms, and autism spectrum disorders. It presents in childhood and is associated with a high risk of sudden cardiac death. All of the variants described are de novo in the different S6 transmembrane segments.1,2
- b)
Isolated LQTS: caused by variants localized to the cytoplasmic linkers and the extreme N and C termini.2
- c)
Syndactyly, psychomotor retardation, and pulmonary hypertension without LQTS: has recently been linked to the p.Arg1024Gly variant.5
- d)
COTS (cardiac-only Timothy syndrome): has been described in 3 families (12 cases) who have one of the following phenotypes from 20 years of age, without extracardiac manifestations: LQTS, hypertrophic cardiomyopathy (HCM), septal defects, and sudden cardiac death.1 p.Arg518Cys and p.Arg518His variants have been identified in the DI–DII linker. There is cosegregation, with complete penetrance and variable expressivity, and none was de novo. An index case, a carrier of the same variant found in our patient, first showed PPCM and LQTS at 25 years of age, had a ventricular septal defect, and developed HCM 7 years later,1 which our patient does not currently have. Recently, another family was described to have HCM, LQTS, and the same variant.4 Functional studies (whole-cell patch clamp) revealed a combination of loss and gain of channel function, with lower global current density and an increased late current and window.1 In addition, there are defects in channel traffic and lower channel concentration in the cell membrane.1 Because this channel is essential for muscular excitation-contraction coupling,1 we consider that it can cause hypertrophy or transient ventricular dysfunction due to poor calcium management.
PPCM is characterized by ventricular dysfunction before or after delivery. Although its cause is unknown, evidence suggests that dilated cardiomyopathy and PPCM patients show a similar prevalence of truncating variants, with truncations in the gene encoding titin the most frequent genetic predisposition in both entities, although other variants are involved as well.6
Our patient showed an association of PPCM and LQTS as the first manifestation of COTS. The presence of a LQTS together with PPCM or HCM should alert physicians to the possible presence of pathogenic variants of CACNA1C.
CONFLICTS OF INTERESTI. Cárdenas-Reyes is an employee of Health in Code SL. L. Monserrat-Iglesias is a shareholder in Health in Code SL.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.01.017.