To the Editor:
Cardiac tamponade is due to extrinsic cardiac compression, generally secondary to pericardial effusion. In rare cases, patients with pleural effusion (PE) can show similar signs and symptoms due to the transmission of the elevated pleural pressure to the heart.1
We report the case of a 34-year-old woman with acquired immunodeficiency syndrome, viral liver disease (hepatitis B, C, and D viruses), right PE and tamponade, in whom a hepatopulmonary syndrome (HPS) was diagnosed.
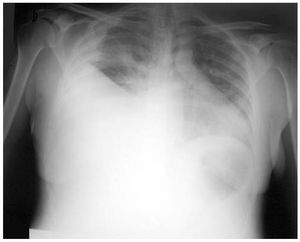
She came to the emergency room with a 7-day history of dyspnea and orthopnea. Her physical condition was poor and she had tachycardia, tachypnea, hypotension (arterial blood pressure, 100/60 mm Hg, which decreased to 80/50 mm Hg in 3 hours), pulsus paradoxus, jugular vein distention, normal heart sounds, decreased intensity of breath sounds in the right lung, and distal edema with pitting. An electrocardiogram revealed sinus tachycardia and low voltages, and chest radiography demonstrated the presence of severe right PE and contralateral mediastinal shift (Figure 1). The patient presented with respiratory failure (pO2, 48 mm Hg), and the most notable findings in the initial laboratory analysis were thrombocytopenia (60 000/μL), PT of 61%, and abnormal liver parameters (aspartate aminotransferase, 159; alanine aminotransferase, 99; alkaline phosphatase, 302; lactate dehydrogenase, 699; and direct bilirubin, 1.7 mg/dL).
Figure 1. Chest radiography. Severe right pleural effusion.
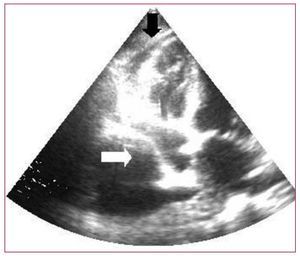
As concomitant pericardial effusion was suspected, an echocardiogram was carried out (Figure 2). The findings included minimal pericardial effusion (2 mm) and severe right PE, systolic and diastolic right atrial collapse, decreased filling velocities, with a respiratory variation of more than 25%, and hepatic vein flow with systolic predominance without reversal of diastolic flow; all these observations are signs of hemodynamic compromise, with no evidence of pericardial effusion.
Figure 2. Echocardiogram. White arrow: right atrial collapse due to pleural effusion. Black arrow: minimal pericardial effusion.
The decision was made to perform thoracocentesis, in which 1000 mL of transudated pleural fluid were drained. Subsequently, there was a marked clinical improvement and normalization of the arterial blood pressure. The echocardiogram was repeated and revealed expansion of the right atrium, without collapse, and normalization of ventricular filling. Pulmonary embolism was ruled out by means of ventilation-perfusion scintigraphy.
The patient remained stable, with oxygen therapy, until 4 days later, when dyspnea, right PE, and right atrial compression reappeared. They resolved once again following the drainage of 800 mL of pleural fluid. Serological and cytological tests and sputum, and blood cultures were negative.
Once the patient was stable, and with the suspicion of HPS, contrast-enhanced echocardiography was performed using agitated saline, which revealed the presence of bubbles in left chambers 5 beats after they were detected in the right chambers. This observation, the improvement in pO2 after the administration of 100% oxygen (pO2, 544 mm Hg) and the chronic liver disease confirm the diagnosis of HPS.
The finding on echocardiography of the collapse of the right chambers is highly sensitive and specific for tamponade.2-5 In most cases, it is due to pericardial effusion, although it can also be caused by severe PE, which transmits the pressure to the pericardium.
There are very few cases of "tamponade" due to PE; of special interest are one involving Budd-Chiari syndrome with bilateral PE6 and certain cases of nonsevere PE in patients who have undergone cardiac surgery.7
Hepatopulmonary syndrome is characterized by chronic liver disease, an increased alveolar-arterial oxygen gradient, and pulmonary vasodilation with low pulmonary vascular resistance8; this implies reduced pressures in the right chambers, which favors their collapse.
Despite the small number of cases of PE that cause right chamber collapse, that is, that behave like a tamponade, we consider that this case helps us to keep in mind that possibility in the differential diagnosis, especially in cases in which there are low intracavitary pressures due to reduced right ventricular afterload, as occurs in HPS. We consider this case to be clinically relevant, not only because of the low incidence of this condition, but because its adjuvant pathophysiological mechanism is HPS.