Preliminary results suggest that high circulating insulin-like growth factor binding protein 2 (IGFBP2) levels are associated with mortality risk in heart failure (HF) patients. As IGFBP2 levels are increased in patients with chronic kidney disease (CKD), which is associated with a higher mortality risk in HF patients, we examined whether IGFBP2 is associated with CKD in HF patients, and whether CKD modifies the prognostic value of this protein in HF patients.
MethodsHF patients (n=686, mean age 66.6 years, 32.7% women) were enrolled and followed up for a median of 3.5 (min-max range: 0.1-6) years. Patients were classified as having CKD with decreased estimated glomerular filtration rate (eGFR <60mL/min/1.73 m2) or as having CKD with nondecreased eGFR (≥ 60mL/min/1.73 m2). Serum IGFBP2 was detected by ELISA.
ResultsIGFBP2 was increased (P <.001) in CKD patients with decreased eGFR (n=290, 42.3%) compared with patients with nondecreased eGFR. IGFBP2 was directly associated with NT-proBNP (P <.001) and inversely associated with eGFR (P <.001), with both associations being independent of confounding factors. IGFBP2 was directly and independently associated with cardiovascular and all-cause death (P <.001) in the whole group of patients, but showed a stronger association with cardiovascular death in CKD patients with decreased eGFR (P for interaction <.05), improving risk prediction in these patients over clinically relevant risk factors.
ConclusionsSerum IGFBP2 is associated with impaired renal function and prognosticates cardiovascular death in patients with HF and CKD with decreased eGFR. Thus, there is an effect modification of CKD on circulating IGFBP2 and on its association with cardiovascular mortality in HF patients.
Keywords
Chronic kidney disease (CKD) is highly prevalent in heart failure (HF) and is associated with a higher risk of mortality and cardiovascular events.1,2 The presence of one condition has a strong influence on the other due to reciprocal interactions between the heart and the kidney that are not completely understood.3 In this regard, as recently pointed out, phenotypic assessment using biomarkers, among other tools, has been proposed to enhance understanding of the interface between the 2 conditions, and to develop optimal strategies for their detection, prevention, diagnosis, and management.4 However, there is still scarce evidence supporting current biomarkers and little progress has been achieved in novel circulating biomarkers useful to risk-stratification in patients with HF and CKD.5
The regulatory hormones belonging to the insulin growth factor (IGF) system have been related to both renal and cardiac diseases. In particular, findings from renal studies show decreased levels of serum IGF-1 in patients with CKD,6 and an association between low levels of IGF-1 and a higher mortality risk in these patients.7 In addition, low serum levels of IGF-1 are associated with a higher risk of all-cause mortality in patients with chronic HF.8 Of note, at least 98% of circulating IGF-1 is bound to IGF binding proteins (IGFBPs), with insulin-like growth factor binding protein 2 (IGFBP2) being one of the most abundant proteins of this family in blood.9 Therefore IGF-1 bioactivity is directly influenced by the IGFBP profile, as an excess of IGFBP2 may reduce the availability of free IGF-1 for its target receptors.9,10 In this context, circulating IGFBP2 has been found to be elevated in patients with CKD,11–14 and in patients with HF, showing an association with a higher risk of mortality in patients with dilated cardiomypathy.15
Taking all the above into consideration, we hypothesized that IGF-1 and IGFBP2 circulating levels may be influenced by CKD in HF patients and may possess prognostic utility especially in patients with both conditions. Therefore, this study was designed to determine the following: first, whether circulating IGF-1 and IGFBP2 are associated with CKD in patients with HF; second, whether these circulating proteins predict mortality in these patients (either cardiovascular or all-cause); and third, whether CKD status modifies the association of these proteins with the risk of cardiovascular or all-cause death in HF patients.
METHODSAll participants gave written informed consent to participate in the study, and the study protocols were approved by the institutional review committees (Clinical Investigation Ethics Committees of Donostia University Hospital and Hospital Universitari Germans Trias i Pujol, Spain). The study conformed to the principles of the 1975 Declaration of Helsinki, as revised in 1983.
Study populationPatients were consecutively enrolled from 2002 to 2010 at 2 multidisciplinary HF units from the Division of Cardiology at the Donostia University Hospital (San Sebastián, Spain) and the Division of Cardiology at the Hospital Universitari Germans Trias i Pujol (Badalona, Spain). The patient population consisted of 686 patients (mean age 66.6 years, range 21-96 years, 32.7% women) and the principal inclusion criterion was HF according to the European Society of Cardiology guidelines irrespective of etiology, together with at least 1 HF hospitalization and/or a reduced left ventricular ejection fraction.16,17 The median time from HF diagnosis until inclusion in the study was 8.0 [interquartile range: 2.0-45.7] months.
Blood samples were taken from the antecubital vein at the time recruitment, kept in ice for 1 to 2hours maximum before processing, aliquoted and kept at−80°C until measured. Additionally, samples were batch-analyzed to minimize differences due to sample processing and storage.
Study outcomesThe main study outcome was death from cardiovascular causes and the second study outcome was all-cause mortality. For additional information see the .
Biochemical studiesSerum IGF-1, IGFBP2, the N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity troponin T (hs-TnT) were measured using ELISA assays. Serum creatinine levels were analyzed using the CREA method and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration 2009 creatinine equation (CKD-EPI).18 The presence of CKD was established according to decreased eGFR (ie, <60mL/min/1.73 m2).19 For additional information see the .
Statistical analysisThe analyses were performed in all patients and in patients classified according to the absence or presence of CKD with decreased eGFR. Normality was demonstrated by the Shapiro-Wilks or Kolmogorov-Smirnov tests. Nonnormally distributed variables were examined after logarithmic transformation. Multivariable linear regression models were performed, with adjustment for covariables significant in univariable analyses. Optimal cutoff values for predicting the outcomes of interest were determined by performing receiver operating characteristic (ROC) curve analysis followed by calculation of the Younden's J statistic.
The cumulative incidence of all-cause death was estimated by the Kaplan-Meier method; unadjusted differences were assessed with log-rank tests. Univariable and multivariable Cox or competing risk regression models (Fine-Gray) with adjustment for noncardiovascular death were used to calculate hazard (HR) or subhazard (SHR) ratios and corresponding 95% confidence intervals for the risk of all-cause death or cardiovascular death, respectively. Patients without outcome were censored at the date of their last follow-up. To determine whether the association of IGFBP2 and IGF-1 with the outcomes of interest differed by the presence of CKD with decreased eGFR, quantitative interaction analyses were performed by Cox or Competitive risk regression analyses in models including IGFBP2 or IGF-1 as continuous variables, CKD with decreased eGFR (yes/no) and their respective interaction terms. In addition, qualitative interaction analyses were performed by Cox or competing risk regression analyses in models including IGFBP-1 or IGF-1 (≤ or >cutoff as determined in ROC analyses), CKD with decreased eGFR (yes/no), and their respective interaction terms.
The baseline characteristics considered as covariables were identified as significant in univariable competing risk regression and Cox analyses for cardiovascular or all-cause death, respectively (), followed by a backward stepwise selection with minimization of the Akaike information criterion and the P value set at .15 for elimination. In patients with nondecreased eGFR, the covariables selected were included in 3 different models due to the low number of cardiovascular death outcomes. In CKD patients with decreased eGFR, a first model was selected according to the previous procedure and a second model was also considered including clinically relevant covariables significant in univariable analyses.
The additional value of the combination of biomarkers for risk prediction of the outcomes of interest was assessed with Harrell's c-statistics and the integrated discrimination (IDI) and the continuous net reclassification (NRI) indexes. Harrell's C estimates were calculated using the Stata package “somersd”. The variances for the NRI and IDI estimates were calculated using bootstrapping (1000 resamples).
Values are expressed as mean±SD or median [interquartile range], and categorical variables as numbers and percentages. Robust standard errors were calculated by using the stata option vce (cluster center), considering the clustering effect of the enrollment of patients in 2 different multidisplicinary units of HF. Statistical significance was set as a 2-sided P value of .05. The statistical analyses were performed by using SPSS (15.0 version) and STATA (12.1 version) software.
For additional information see the .
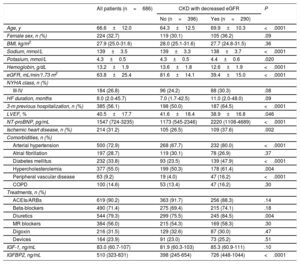
RESULTSPatient characteristicsThe patient population consisted of 686 patients, (mean age 66.6 years, range 21-96 years, 32.7% women). Table 1 shows the clinical and echocardiographic characteristics of all HF patients and of patients classified in 2 groups: patients with CKD as defined by a decreased eGFR (ie, <60mL/min/1.73 m2) (n=290) and patients with nondecreased eGFR (ie, ≥ 60mL/min/1.73 m2) (n=396). Compared with patients with nondecreased eGFR, CKD patients were older, had lower levels of serum sodium, hemoglobin and reduced left ventricular ejection fraction, and showed increased levels of serum potassium, NT-proBNP and a higher rate of hospitalizations in the previous 3 months, ischemic heart disease, hypertension, diabetes mellitus, hypercholesterolemia, peripheral vascular disease and diuretic treatment (table 1).
Clinical characteristics of heart failure patients categorized according to the absence or presence of CKD with decreased eGFR (< 60mL/min/1.73 m2) at baseline
| All patients (n=686) | CKD with decreased eGFR | P | ||
|---|---|---|---|---|
| No (n=396) | Yes (n=290) | |||
| Age, y | 66.6±12.0 | 64.3±12.5 | 69.9±10.3 | <.0001 |
| Female sex, n (%) | 224 (32.7) | 119 (30.1) | 105 (36.2) | .09 |
| BMI, kg/m2 | 27.9 (25.0-31.6) | 28.0 (25.1-31.6) | 27.7 (24.8-31.5) | .36 |
| Sodium, mmol/L | 139±3.5 | 139±3.3 | 138±3.7 | <.0001 |
| Potasium, mmol/L | 4.3±0.5 | 4.3±0.5 | 4.4±0.6 | .020 |
| Hemoglobin, g/dL | 13.2±1.9 | 13.6±1.8 | 12.6±1.9 | <.0001 |
| eGFR, mL/min/1.73 m2 | 63.8±25.4 | 81.6±14.1 | 39.4±15.0 | <.0001 |
| NYHA class, n (%) | ||||
| III-IV | 184 (26.8) | 96 (24.2) | 88 (30.3) | .08 |
| HF duration, months | 8.0 (2.0-45.7) | 7.0 (1.7-42.5) | 11.0 (2.0-48.0) | .09 |
| 3-m previous hospitalization, n (%) | 385 (56.1) | 198 (50.0) | 187 (64.5) | <.0001 |
| LVEF, % | 40.5±17.7 | 41.6±18.4 | 38.9±16.8 | .046 |
| NT-proBNP, pg/mL | 1547 (724-3235) | 1173 (545-2346) | 2220 (1108-4689) | <.0001 |
| Ischemic heart disease, n (%) | 214 (31.2) | 105 (26.5) | 109 (37.6) | .002 |
| Comorbidities, n (%) | ||||
| Arterial hypertension | 500 (72.9) | 268 (67.7) | 232 (80.0) | <.0001 |
| Atrial fibrillation | 197 (28.7) | 119 (30.1) | 78 (26.9) | .37 |
| Diabetes mellitus | 232 (33.8) | 93 (23.5) | 139 (47.9) | <.0001 |
| Hypercholesterolemia | 377 (55.0) | 199 (50.3) | 178 (61.4) | .004 |
| Peripheral vascular disease | 63 (9.2) | 19 (4.0) | 47 (16.2) | <.0001 |
| COPD | 100 (14.6) | 53 (13.4) | 47 (16.2) | .30 |
| Treatments, n (%) | ||||
| ACEIs/ARBs | 619 (90.2) | 363 (91.7) | 256 (88.3) | .14 |
| Beta-blockers | 490 (71.4) | 275 (69.4) | 215 (74.1) | .18 |
| Diuretics | 544 (79.3) | 299 (75.5) | 245 (84.5) | .004 |
| MR blockers | 384 (56.0) | 215 (54.3) | 169 (58.3) | .30 |
| Digoxin | 216 (31.5) | 129 (32.6) | 87 (30.0) | .47 |
| Devices | 164 (23.9) | 91 (23.0) | 73 (25.2) | .51 |
| IGF-1, ng/mL | 83.0 (60.7-107) | 81.9 (60.3-103) | 85.3 (60.9-111) | .10 |
| IGFBP2, ng/mL | 510 (323-831) | 398 (245-654) | 726 (448-1044) | <.0001 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blockers; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; IGF, insulin growth factor; IGFBP2, insulin-like growth factor binding protein 2; LVEF, left ventricular ejection fraction; MR, mineralocorticoid receptor; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
Quantitative variables are expressed as mean±standard deviation or as median (interquartile range). Categorical variables are expressed as numbers (percentages).
Serum IGF-1 levels were similar in the 2 groups, but serum IGFBP2 was higher in CKD patients with decreased eGFR than in those with nondecreased eGFR (table 1).
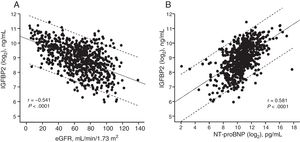
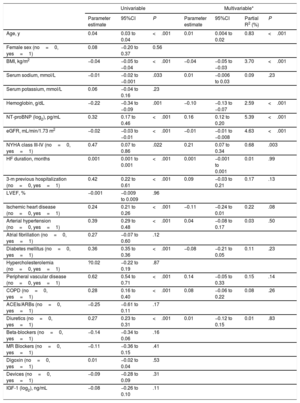
Analyses of associationsIn all patients, IGFBP2 was inversely correlated with eGFR (figure 1A) and directly correlated with NT-proBNP (figure 1B). Multiple regression analyses in table 2 showed that eGFR and NT-proBNP were predictors of the increase in IGFBP2 independently of age, body mass index, serum sodium, hemoglobin, New York Heart Association (NYHA) class, HF duration, hospitalization in the previous 3 months, the presence of ischemic heart disease, hypertension, diabetes mellitus, peripheral vascular disease, chronic obstructive pulmonary disease and diuretic treatment. Interestingly, among the previously mentioned variables, eGFR and NT-proBNP explained the highest percentages of the IGFBP2 variance (table 2).
Linear regression diagrams between the estimated glomerular filtration rate (eGFR) and insulin-like growth factor binding protein 2 (IGFBP2) (log2) (y=10.3-0.02x) (A) and between the N-terminal pro–B-type natriuretic peptide (NT-proBNP) (log2) and IGFBP2 (log2) (y=0.32x-5.62) (B) in all heart failure patients. Dotted lines are the 95% prediction limits.
Univariable and multivariable linear regression analyses for the association of IGFBP2 (log2) with clinical variables
| Univariable | Multivariable* | ||||||
|---|---|---|---|---|---|---|---|
| Parameter estimate | 95%CI | P | Parameter estimate | 95%CI | Partial R2 (%) | P | |
| Age, y | 0.04 | 0.03 to 0.04 | <.001 | 0.01 | 0.004 to 0.02 | 0.83 | <.001 |
| Female sex (no=0, yes=1) | 0.08 | −0.20 to 0.37 | 0.56 | ||||
| BMI, kg/m2 | −0.04 | −0.05 to −0.04 | <.001 | −0.04 | −0.05 to −0.03 | 3.70 | <.001 |
| Serum sodium, mmol/L | −0.01 | −0.02 to −0.001 | .033 | 0.01 | −0.006 to 0.03 | 0.09 | .23 |
| Serum potassium, mmol/L | 0.06 | −0.04 to 0.16 | .23 | ||||
| Hemoglobin, g/dL | −0.22 | −0.34 to −0.09 | .001 | −0.10 | −0.13 to −0.07 | 2.59 | <.001 |
| NT-proBNP (log2), pg/mL | 0.32 | 0.17 to 0.46 | <.001 | 0.16 | 0.12 to 0.20 | 5.39 | <.001 |
| eGFR, mL/min/1.73 m2 | −0.02 | −0.03 to −0.01 | <.001 | −0.01 | −0.01 to −0.008 | 4.63 | <.001 |
| NYHA class III-IV (no=0, yes=1) | 0.47 | 0.07 to 0.86 | .022 | 0.21 | 0.07 to 0.34 | 0.68 | .003 |
| HF duration, months | 0.001 | 0.001 to 0.001 | <.001 | 0.001 | −0.001 to 0.001 | 0.01 | .99 |
| 3-m previous hospitalization (no=0, yes=1) | 0.42 | 0.22 to 0.61 | <.001 | 0.09 | −0.03 to 0.21 | 0.17 | .13 |
| LVEF, % | −0.001 | −0.009 to 0.009 | .96 | ||||
| Ischemic heart disease (no=0, yes=1) | 0.24 | 0.21 to 0.26 | <.001 | −0.11 | −0.24 to 0.01 | 0.22 | .08 |
| Arterial hypertension (no=0, yes=1) | 0.39 | 0.29 to 0.48 | <.001 | 0.04 | −0.08 to 0.17 | 0.03 | .50 |
| Atrial fibrillation (no=0, yes=1) | 0.27 | −0.07 to 0.60 | .12 | ||||
| Diabetes mellitus (no=0, yes=1) | 0.36 | 0.35 to 0.36 | <.001 | −0.08 | −0.21 to 0.05 | 0.11 | .23 |
| Hypercholesterolemia (no=0, yes=1) | ?0.02 | −0.22 to 0.19 | .87 | ||||
| Peripheral vascular disease (no=0, yes=1) | 0.62 | 0.54 to 0.71 | <.001 | 0.14 | −0.05 to 0.33 | 0.15 | .14 |
| COPD (no=0, yes=1) | 0.28 | 0.16 to 0.40 | <.001 | 0.08 | −0.06 to 0.22 | 0.08 | .26 |
| ACEIs/ARBs (no=0, yes=1) | −0.25 | −0.61 to 0.11 | .17 | ||||
| Diuretics (no=0, yes=1) | 0.27 | 0.23 to 0.31 | <.001 | 0.01 | −0.12 to 0.15 | 0.01 | .83 |
| Beta-blockers (no=0, yes=1) | −0.14 | −0.34 to 0.06 | .16 | ||||
| MR Blockers (no=0, yes=1) | −0.11 | −0.36 to 0.15 | .41 | ||||
| Digoxin (no=0, yes=1) | 0.01 | −0.02 to 0.04 | .53 | ||||
| Devices (no=0, yes=1) | −0.09 | −0.28 to 0.09 | .31 | ||||
| IGF-1 (log2), ng/mL | −0.08 | −0.26 to 0.10 | .11 | ||||
95%CI, 95% confidence interval; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blockers; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; IGF, insulin growth factor; IGFBP2, insulin-like growth factor binding protein 2; LVEF, left ventricular ejection fraction; MR, mineralocorticoid receptor; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
On the other hand, circulating IGF-1 levels showed associations with age, female sex, NYHA class, hospitalization in the previous 3 months and treatment with beta-blockers and digoxin (). However, this protein did not show univariable associations with either eGFR or NT-proBNP ().
Survival analysesDuring a median follow-up of 3.5 (min-max range: 0.1-6) years, cardiovascular death occurred in 98 patients (14.3%) and all-cause death occurred in 177 patients (25.8%). In patients with nondecreased eGFR, cardiovascular death and all-cause death occurred in 36 (9.1%) and 83 (21.0%) patients, respectively. In the CKD group, cardiovascular death and all-cause death occurred in 62 (21.4%) and 94 (32.4%) patients, respectively. Longitudinal analyses showed that patients with CKD (and decreased eGFR) had a higher risk of cardiovascular death and all-cause death than patients with nondecreased eGFR ().
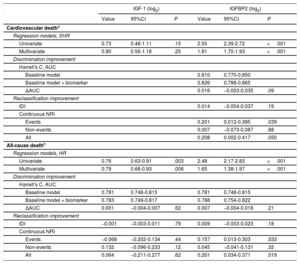
Multivariable competing risk and Cox regression analyses showed that whereas increased IGFBP2 and decreased IGF-1 were associated with all-cause death independently of a baseline model including age, body mass index, NYHA class, eGFR, NT-proBNP (log2), hemoglobin, chronic obstructive pulmonary disease, diabetes mellitus and peripheral vasculopathy, only IGFBP2 was associated with cardiovascular death independently of age, NYHA class, eGFR, NT-proBNP (log2), hemoglobin, chronic obstructive pulmonary disease, ischemic cardiomyopathy, and diuretic treatment (table 3). However, no significant prognostic improvement was observed for IGFBP2 in addition to any of the baseline models considered in the discrimination and IDI reclassification analyses. Nonetheless, IGFBP2 improved discrimination for both outcomes according to NRI values (P ≤ .05, table 3).
Associations of IGF-1 and IGFBP2 levels with the outcomes of interest and added predictive value
| IGF-1 (log2) | IGFBP2 (log2) | |||||
|---|---|---|---|---|---|---|
| Value | 95%CI | P | Value | 95%CI | P | |
| Cardiovascular deatha | ||||||
| Regression models, SHR | ||||||
| Univariate | 0.73 | 0.48-1.11 | .15 | 2.55 | 2.39-2.72 | <.001 |
| Multivariate | 0.80 | 0.56-1.16 | .25 | 1.81 | 1.70-1.93 | <.001 |
| Discrimination improvement | ||||||
| Harrell's C, AUC | ||||||
| Baseline model | 0.810 | 0.770-0.850 | ||||
| Baseline model + biomarker | 0.826 | 0.788-0.865 | ||||
| ΔAUC | 0.016 | −0.003-0.035 | .09 | |||
| Reclassification improvement | ||||||
| IDI | 0.014 | −0.004-0.037 | .19 | |||
| Continuous NRI | ||||||
| Events | 0.201 | 0.012-0.395 | .039 | |||
| Non-events | 0.007 | −0.073-0.087 | .88 | |||
| All | 0.208 | 0.002-0.417 | .050 | |||
| All-cause deathb | ||||||
| Regression models, HR | ||||||
| Univariate | 0.76 | 0.63-0.91 | .003 | 2.48 | 2.17-2.83 | <.001 |
| Multivariate | 0.79 | 0.66-0.93 | .006 | 1.65 | 1.38-1.97 | <.001 |
| Discrimination improvement | ||||||
| Harrell's C, AUC | ||||||
| Baseline model | 0.781 | 0.748-0.815 | 0.781 | 0.748-0.815 | ||
| Baseline model + biomarker | 0.783 | 0.749-0.817 | 0.788 | 0.754-0.822 | ||
| ΔAUC | 0.001 | −0.004-0.007 | .62 | 0.007 | −0.004-0.018 | .21 |
| Reclassification improvement | ||||||
| IDI | −0.001 | −0.003-0.011 | .79 | 0.009 | −0.003-0.023 | .18 |
| Continuous NRI | ||||||
| Events | −0.068 | −0.202-0.134 | .44 | 0.157 | 0.013-0.303 | .033 |
| Non-events | 0.132 | −0.096-0.233 | .12 | 0.045 | −0.041-0.131 | .32 |
| All | 0.064 | −0.211-0.277 | .62 | 0.201 | 0.034-0.371 | .019 |
95%CI, 95% confidence interval; AUC, area under the receiver operating characteristic curve; IDI, integrated discrimination index; IGF, insulin growth factor; IGFBP2, insulin-like growth factor binding protein 2; NRI, net reclassification index.
Hazard (HR) and sub-hazard (SHR) ratios are effects sizes for a doubling of serum IGF-1 and IGFBP2.
Baseline model: age, New York Heart Association class, estimated glomerular filtration rate, N-terminal pro–B-type natriuretic peptide (log2), hemoglobin, chronic obstructive pulmonary disease, ischemic cardiomyopathy and treatment with diuretics. The N in the final model was equal to 684.
Baseline model: age, body mass index, New York Heart Association class, estimated glomerular filtration rate, N-terminal pro–B-type natriuretic peptide (log2), hemoglobin, chronic obstructive pulmonary disease, diabetes mellitus and peripheral vasculopathy. The N in the final model was equal to 683.
Cutoff values to predict cardiovascular and all-cause death for IGFBP2 and IGF-1 were calculated by ROC analyses in all patients (). With these thresholds, 237 patients (34.5%) were classified as the high IGFBP2 subgroup and 316 patients (46.1%) were classified as the low IGF-1 subgroup. Interaction analyses were performed to assess whether the associations among IGF-1 or IGFBP2 with the outcomes of interest could be influenced by the presence of CKD (with decreased eGFR), considering the levels of these proteins as continuous or categorical (> or <cutoff as determined in ROC analyses) variables in quantitative and qualitative analyses, respectively. In adjusted analyses controlling for the previously mentioned baseline models (selected for each outcome of interest in the whole population), a significant interaction was observed for the influence of CKD on the association between IGFBP2 and cardiovascular death in quantitative (P=.038) and qualitative (P <.001) analyses (chi-square=7.5, df=2, omnibus P=.023 for the overall interaction effect in the qualitative analysis). In fact, whereas in patients with nondecreased eGFR, high levels of IGFBP2 (above the cutoff=686 ng/mL) were associated with a subhazard ratio of 1.97 (95% confidence interval [95%CI], 1.58-2.47; P <.001) for the risk of cardiovascular death (), in CKD patients, high levels of IGFBP2 were associated with a subhazard ratio of 6.48 (95%CI, 3.21-13.0; P <.001) (). In addition, shows that patients with both high levels of IGFBP2 and an eGFR <30mL/min/1.73 m2 had the highest rate of cardiovascular death (40.6%). Regarding the association between IGFBP2 and all-cause death, interactions were not observed in either qualitative (P=.78) or quantitative (P=.63) analyses.
No significant interactions were found for the influence of CKD on the association between IGF-1 and the outcomes of interest in either quantitative (cardiovascular death: P=.55; all-cause death: P=.99) or qualitative (cardiovascular death: P=.72; all-cause death: P=.87) analyses.
Given that CKD status can influence the potential prognostic value of IGFBP2, longitudinal analyses to evaluate the association of IGFBP2 with cardiovascular death (as the outcome for which an interaction has been identified) have been performed separately in patients with nondecreased eGFR and in CKD patients (with decreased eGFR).
Prognostic value of IGFBP2 in patients with nondecreased eGFRROC analysis showed that the AUC for IGFBP2 to predict cardiovascular death in patients with nondecreased eGFR was 0.674 (95%CI: 0.585-0.763, P=.001) and the best cutoff value was 564 ng/mL with a sensitivity of 61.1% and a specificity of 69.7%. With this threshold, 131 patients (33.1%) were included in the high IGFBP2 subgroup.
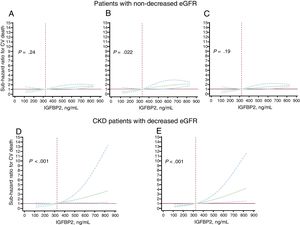
Adjusted analyses showed independent associations of the continuous and the categorical IGFBP2 variable (below or above 564 ng/mL) with cardiovascular death in patients with nondecreased eGFR (figure 2A-C, ). Nonetheless, no significant prognostic improvement was observed by adding the continuous or categorical IGFBP2 variables to any of the baseline models considered in the discrimination and IDI reclassification analyses, although significant NRI values were found for the categorical IGFBP2 variable ().
Subhazard ratios (solid black lines) and 95% confidence intervals (dotted blue lines) for the association between baseline insulin-like growth factor binding protein 2 (IGFBP2) and cardiovascular (CV) death occurrence in heart failure patients with nondecreased estimated glomerular filtration rate (eGFR ≥ 60mL/min/1.73 m2) after adjustment by age and sex (A), serum sodium and NYHA class (B) and ischemic heart disease and N-terminal pro–B-type natriuretic peptide (NT-proBNP) (log2) (C), and in patients with HF and chronic kidney disease (CKD) with decreased eGFR (< 60mL/min/1.73 m2) after adjustment by age, NYHA, ischemic cardiomyopathy, eGFR and NT-proBNP (log2) (D), and after adjustment by hemoglobin, diabetes mellitus and treatment with angiotensin converting enzyme inhibitor or angiotensin II type 1 receptor blockers (E). In each model, the baseline IGFBP2 levels were modeled with restricted cubic splines with 3 knots in the competing risk regression models (Fine-Gray) where the competing event was noncardiovascular death. The reference level was set at the 25th percentile in the whole population (324 ng/mL, dotted vertical red line). All graphs were truncated at the 75th percentile (831 ng/mL). The horizontal red line indicates SHR=1.
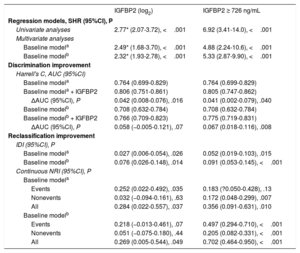
ROC analysis showed that the AUC for IGFBP2 to predict cardiovascular death in CKD patients was 0.730 (95%CI, 0.667-0.794; P <.001) and that the best cutoff value was 726 ng/mL with a sensitivity of 83.9% and a specificity of 59.3%. With this threshold, 145 patients (50.0%) were included in the high IGFBP2 subgroup.
Adjusted analyses showed associations of the continuous and the categorical IGFBP2 variable (below or above 726 ng/mL) with cardiovascular death in CKD patients after adjustment for covariables both in model 1 (age, NYHA, ischemic cardiomyopathy, eGFR and NT-proBNP) and in model 2 (hemoglobin, diabetes mellitus, treatment with ACEIs/ARBs) (figure 2C, D, table 4). In addition, Harrell's c-statistics indicated a significant discrimination improvement in the prediction of cardiovascular death after the addition of the continuous and the categorical IGFBP2 variables on top of the baseline models (table 4). Moreover, the IDI and continuous NRI indexes indicated that the addition of IGFBP2 to the baseline models improved their ability to predict cardiovascular death in CKD patients (table 4).
Associations of IGFBP2 and added predictive value for cardiovascular death in CKD patients with decreased eGFR (< 60mL/min/1.73 m2)
| IGFBP2 (log2) | IGFBP2 ≥ 726 ng/mL | |
|---|---|---|
| Regression models, SHR (95%CI), P | ||
| Univariate analyses | 2.77* (2.07-3.72), <.001 | 6.92 (3.41-14.0), <.001 |
| Multivariate analyses | ||
| Baseline modela | 2.49* (1.68-3.70), <.001 | 4.88 (2.24-10.6), <.001 |
| Baseline modelb | 2.32* (1.93-2.78), <.001 | 5.33 (2.87-9.90), <.001 |
| Discrimination improvement | ||
| Harrell's C, AUC (95%CI) | ||
| Baseline modela | 0.764 (0.699-0.829) | 0.764 (0.699-0.829) |
| Baseline modela + IGFBP2 | 0.806 (0.751-0.861) | 0.805 (0.747-0.862) |
| ΔAUC (95%CI), P | 0.042 (0.008-0.076), .016 | 0.041 (0.002-0.079), .040 |
| Baseline modelb | 0.708 (0.632-0.784) | 0.708 (0.632-0.784) |
| Baseline modelb + IGFBP2 | 0.766 (0.709-0.823) | 0.775 (0.719-0.831) |
| ΔAUC (95%CI), P | 0.058 (−0.005-0.121), .07 | 0.067 (0.018-0.116), .008 |
| Reclassification improvement | ||
| IDI (95%CI), P | ||
| Baseline modela | 0.027 (0.006-0.054), .026 | 0.052 (0.019-0.103), .015 |
| Baseline modelb | 0.076 (0.026-0.148), .014 | 0.091 (0.053-0.145), <.001 |
| Continuous NRI (95%CI), P | ||
| Baseline modela | ||
| Events | 0.252 (0.022-0.492), .035 | 0.183 (?0.050-0.428), .13 |
| Nonevents | 0.032 (−0.094-0.161), .63 | 0.172 (0.048-0.299), .007 |
| All | 0.284 (0.022-0.557), .037 | 0.356 (0.091-0.631), .010 |
| Baseline modelb | ||
| Events | 0.218 (−0.013-0.461), .07 | 0.497 (0.294-0.710), <.001 |
| Nonevents | 0.051 (−0.075-0.180), .44 | 0.205 (0.082-0.331), <.001 |
| All | 0.269 (0.005-0.544), .049 | 0.702 (0.464-0.950), <.001 |
95%CI, 95% confidence interval; AUC, area under-the-curve; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; IDI, integrated discrimination index; IGFBP2, insulin-like growth factor binding protein 2; NRI, net reclassification index.
The major findings of this study are the following: a) In HF patients, whereas IGF-1 remained unchanged, IGFBP2 increased in CKD patients (with decreased eGFR) compared with patients with nondecreased eGFR; b) IGFBP2 showed independent inverse and direct associations with eGFR and NT-proBNP, respectively; c) increased IGFBP2 was independently associated with a higher risk of cardiovascular and all-cause mortality in all HF patients; d) the association of IGFBP2 with cardiovascular death was significantly stronger in patients with HF and CKD (with decreased eGFR), increasing the prognostic value above and beyond relevant clinical variables (including cardiac and renal-related parameters).
Our findings indicate that IGFBP2 serum levels are particularly increased in patients with HF and CKD (ie, with decreased eGFR) compared with HF patients with nondecreased eGFR, whereas no significant changes were found in circulating IGF-1. Of note, previous studies have already described an increment in serum IGFBP2, with unchanged IGF-1 levels, in patients with renal disease, showing inverse correlations between IGFBP2 and eGFR.14,20 In addition, a recent experimental study confirmed the association of high circulating levels of IGFBP2 and renal injury.21 However, the cause for the rise in serum IGFPB2 in HF patients, particularly in those with CKD, is unknown. In this regard, it has been reported that, in humans, IGFBP2 is mainly produced by the heart and the liver.22 Experimental studies show that the increment in circulating IGFBP2 found in nephrotic rats is caused, at least in part, by an increase in the hepatic synthesis of this binding protein.12 In addition, increased renal expression of IGFBP2 has been reported in experimental and human kidney disease.23 In this context, whether the increment in circulating IGFBP2 in patients with HF and CKD is related to higher cardiac, renal and/or hepatic synthesis deserves further studies. Alternatively, increased spillover of IGFBP2 into the circulation from other tissue sources may also contribute to the association of IGFBP2 with impaired renal function reported in this study. On the other hand, even though the size of IGFBP2 is lower than the normal glomerular pore size (approximately 10nm), we cannot exclude the possibility that the accumulation of IGFBP2 in serum with the decline of eGFR reflects renal retention of IGFBP2 complexes.
Interestingly, we have found that decreased IGF-1 and increased IGFBP2 levels are associated with a higher risk of mortality in HF patients. These findings confirm previous observations linking low serum levels of IGF-1 and a higher mortality risk in patients with chronic HF and reduced ejection fraction.8 In addition, the association between high IGFBP2 and mortality risk has been previously reported in 90 patients with idiopathic dilated cardiomyopathy.15 We further confirm and expand these observations in a larger cohort of patients with HF of different etiologies, describing for the first time that the prognostic ability of IGFBP2 to predict cardiovascular death is stronger in patients with HF and CKD (with decreased eGFR). In particular, IGFBP2 improves the prognostic accuracy of baseline models including clinically relevant cardiac and renal-related biomarkers such as NT-proBNP and eGFR in patients with HF and CKD. Of note, the prognostic usefulness of other circulating members of the IGFBP family has been studied in cardiac patients with conflicting results. In particular, IGFBP1 levels are increased in HF patients although without showing any prognostic value, and studies evaluating IGFBP3 as a cardiovascular biomarker show controversial findings.24 In addition, IGFBP7 is associated with a higher risk of all-cause mortality and of HF hospitalization in patients with HF with preserved ejection fraction, although its prognostic value is diminished in the presence of relevant cardiovascular risk factors.25 Collectively, our results suggest that IGFBP2 meets the necessary requirements26 to be considered a potential biomarker of cardiovascular risk in HF patients, particularly in patients with HF and CKD (with decreased eGFR): a) Association of the marker alone with the outcome of interest; b) association of the marker with the outcome of interest after statistical adjustment for standard risk factors; and c) significant improvement of a model containing the standard risk factors after addition of the novel marker. Nonetheless, to confirm the value of IGFBP2 as a clinically useful biomarker, the development of standardized assays would be necessary to ascertain reproducibility and allow its availability in clinical practice. In addition, further studies are necessary to perform prospective validations in independent cohorts, assessment of usefulness for patient management and outcomes, and ultimately, cost-effectiveness.26
The potential pathophysiological mechanisms linking IGFBP2 with HF remain unknown. We observed a direct correlation between IGFBP2 and NT-proBNP in HF patients, with this association being independent of eGFR among other relevant clinical variables. IGFBP2 has the ability to bind IGF-1 and IGF-2, primarily inhibiting IGF actions in the tissue.10 Considering that IGF-1 exerts cardiorenal protective actions27,28 and that IGF-1-IGFBP2 complexes may be more abundant in conditions of CKD and HF,15,29 it is tempting to speculate that IGFBP2 might inhibit IGF-1 organ-protective bioactivity, having a detrimental impact on cardiac and renal function and on HF prognosis.
Our investigation has some limitations. First, this study reflects the experience of only 2 centers and is limited mainly to Caucasians. Second, we focused our study only on IGF-1 and IGFBP2, but it would be interesting to evaluate other members of the IGF and IGFBP families in HF patients from this study. In addition, this study would have been enriched by evaluation of IGF-1 and IGFBP2 in control participants. Third, subgroup analyses are commonly considered as exploratory with limited generalizability. Moreover, the reduction in sample size and frequency of the outcomes of interest due to categorization in subgroups may have affected the statistical power to assess IGFBP2 prognostic value, particularly in patients with HF and nondecreased eGFR. Therefore, further analyses in independent cohorts are needed to confirm these findings. Fourth, potential problems related to multiplicity could have influenced the findings obtained. Fifth, as albumin levels in urine and renal imaging assessment were not available in patients in this study, we cannot exclude the possibility of structural renal damage in those patients with nondecreased eGFR, who could be in stages 1 and 2 of CKD. Finally, because they are descriptive in nature, the associations found between circulating IGFBP2, cardiac and renal function and clinical outcomes do not establish causality.
In conclusion, serum IGFBP2 is independently associated with decreased eGFR and with a higher risk of mortality in HF patients, showing a higher prognostic value for cardiovascular death in those patients in whom HF and CKD (with decreased eGFR) coexist. Thus, there is an effect modification of CKD on the association of IGFBP2 with cardiovascular mortality in HF patients. In addition, serum IGFBP2 improves risk prediction when added to traditional cardiovascular and renal risk factors in patients with HF and CKD. Therefore, IGFBP2 emerges as a promising biomarker of the cardiorenal interaction in HF patients. However, there is a need to investigate the pathophysiological mechanisms linking increased IGFBP2 with the decline in eGFR, as well as its association with poor cardiovascular outcome in larger and independent cohorts of HF patients with and without CKD. It is tempting to speculate that these mechanisms may provide useful information on the complex interactions between the heart and the kidney in HF patients.
FUNDINGThis work was supported by the Dirección General de Industria, Energía e Innovación, Gobierno de Navarra, Spain (MINERVA; codes 0011-1411-2018-000053 and 0011-1411-2018-000044), the Ministry of Economy and Competitiveness, Spain (Instituto de Salud Carlos III grants CB16/11/00483), and the European Commission FP7 Programme (HOMAGE project 2012-305507).
CONFLICTS OF INTERESTNone declared.
HF mortality and morbidity are unacceptably high. CKD is a prevalent comorbidity in HF that further increases mortality risk. The presence of one condition has a strong influence on the other due to reciprocal interactions between the heart and the kidney that are not completely understood. We provide evidence supporting IGFBP2 as a biomarker that could help to improve risk stratification, leading to better management of patients with HF and CKD.
WHAT DOES THIS STUDY ADD?The current article describes for the first time that increased serum IGFBP2 is associated with impaired renal function and with biomarkers of cardiac dysfunction in patients with HF, providing additional prognostic information on mortality risk, over and above traditional cardiovascular and renal risks factors, especially in those patients with both HF and CKD (with decreased eGFR). We therefore believe that our findings support the notion that IGFBP2 is a promising biomarker of cardiorenal interaction in HF patients.
The authors thank Sonia Martínez for her valuable technical assistance.
Supplementary data associated with this article can be found in the online version available at http://dx.doi.org/10.1016/j.rec.2019.10.012