Cardiovascular magnetic resonance plays an increasingly important role in routine cardiology clinical practice. It is a versatile imaging modality that allows highly accurate, broad and in-depth assessment of cardiac function and structure and provides information on pertinent clinical questions in diseases such as ischemic heart disease, nonischemic cardiomyopathies, and heart failure, as well as allowing unique indications, such as the assessment and quantification of myocardial iron overload or infiltration. Increasing evidence for the role of cardiovascular magnetic resonance, together with the spread of knowledge and skill outside expert centers, has afforded greater access for patients and wider clinical experience. This review provides a snapshot of cardiovascular magnetic resonance in modern clinical practice by linking image acquisition and postprocessing with effective delivery of the clinical meaning.
Keywords
Standardized cardiovascular magnetic resonance (CMR) acquisition protocols are designed to purposefully address clinical questions. Contemporary use of CMR in routine clinical practice has been shaped through the efforts of standardization from image acquisition to post-processing,1,2 to obtain the wealth of clinical information that affects patient management and outcomes, supporting reimbursement.3 Extensive and lengthy imaging acquisitions and overly descriptive CMR reports have been scrutinized to clinically relevant outputs, with essential measurements and information serving to answer the clinical question. Two imaging protocols will clarify most pertinent questions in modern clinical cardiology workflows:
- 1.
“Left ventricular (LV) function and scar/fibrosis protocol”. This protocol assesses cardiac volumes and function by cine imaging, and the presence, extent and the type of scar/fibrosis by late gadolinium enhancement (LGE) (Figure 1A).
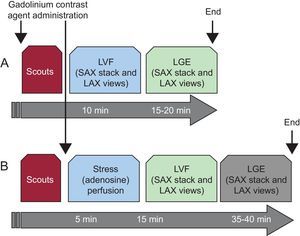
Figure 1.Basic cardiovascular magnetic resonance protocols of everyday clinical routine based on the robustness and maximized information output of imaging applications. A: Left ventricular function and late gadolinium enhancement represents a standard assessment of cardiac function and structure and recognition of underlying cardiomyopathy, based on the presence and pattern of late gadolinium enhancement. B: Addition of myocardial stress (adenosine) perfusion to the basic scheme support assessment of myocardial ischemia when mandated by the typical angina symptoms. Omission of rest perfusion is based on the negligible additional information in the presence of late gadolinium enhancement, which reduces the scan time and contrast dose. LAX, long axis; LGE, late gadolinium enhancement; LVF, left ventricular function; SAX, short axis.
(0.13MB). - 2.
“Myocardial stress perfusion protocol”. This protocol is added to the above basic framework when required by the symptoms, suggesting the presence of myocardial ischemia or, where relevant, the need for guidance by coronary intervention (Figure 1B).
Because of their ease and robustness, these 2 CMR protocols can be performed in most clinical departments within 20 and 45minutes, respectively, delivering diagnostic images in nearly all patients except for a small number not able to benefit from magnetic resonance imaging (MRI), either due to contraindications (such as medical devices) or large body size. Such short imaging times increase patient comfort, efficient clinical throughput, and experience in image acquisition and interpretation. A handful of meaningful departures from this basic scheme together with their clinical intent are included in the second part of this review.
CARDIAC VOLUMES AND FUNCTIONUnlimited by acquisition windows, CMR can provide an overview of cardiac morphology, as well as of the relationships of the cardiac chambers with the great vessels and other thoracic structures. The mainstay of radiological practice, the black-blood sequences, where the blood signal is suppressed to highlight the vascular and myocardial structures, have been replaced by faster gradient echo sequences, and eventually, by balanced sequences (steady-state free precession sequences). These modern sequences allow rapid acquisition, obtaining multiple consecutive images that can be displayed in “still” or “moving-cine” mode to show cardiac movement.
Cine (balanced) imaging is the gold standard of the quantification of biventricular volumes and function, as well as LV mass, overcoming the limitations of echocardiography such as poor acoustic window or geometrical assumptions that lead to inaccuracies and lower reproducibility of the measurements. An excellent definition of endocardial and epicardial borders translates into higher reliability of the obtained values, with excellent interobserver and intraobserver variability (interobserver variability of 4.4% for ejection fraction, 6.3% for diastolic volume, and 8.6% for systolic volume; intraobserver variability 4.0%, 3.6% and 6.5%, respectively).4 In addition to chamber quantification, cine imaging allows inspection of cardiac structure and regional wall motion, accurately and reproducibly.5 Because these parameters provide the basis of clinical management decisions, such as establishing diagnosis (eg, dilative cardiomyopathy6) or serving as a guide to treatment (eg, device therapy7), the accuracy of the measurements is key. A further advantage is assessment of the right ventricle, which is a challenge in echocardiography due to its complex shape, which does not adapt to any geometrical model.
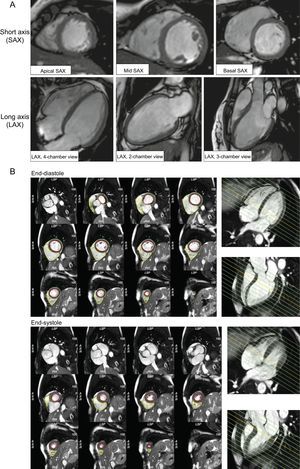
Cine images are obtained in a routine adult patient, as shown in Figure 2:
- 1.
A contiguous stack of short axis slices, defined by the centerline long axis of the LV
- 2.
Perpendicular long axis views (2-, 3- and 4-chamber views).
Assessment of left ventricular function and structure. A: Basic geometries in assessment of cardiac function and structure follow the standard recommendations in cardiovascular imaging based on the 17-segment model, and imaging planes in short-axis views (short axis, apical, mid-ventricular, basal) and long-axis (long axis, 2-, 3- and 4-chamber) views. These same geometries will be also used for perfusion and late gadolinium enhancement modules. This allows a side-to-side comparison of cine images and wall motion abnormalities with scar images. B: A full short axis stack of slices completely covering from the base of both ventricles to the cardiac apex is required for manual or automated tracing of the endocardial and epicardial ventricular border. Left ventricular and right ventricular postprocessing based on Society for Cardiovascular Magnetic Resonance recommendation in diastole and systole, based on an example using CVI42®. Using long axis-view side-by-side supports delineation of the structures in the basal slices reducing the source of a common error in cardiac volume quantification.
The coverage of the short axis stack will include both ventricles in their entirety. This acquisition scheme or geometry is followed in the subsequent LGE acquisition, allowing for side-by-side assessment of findings. Standardized post-processing recommendations outline the principles of quantification, which are adopted by approved post-processing software,2 and allow longitudinal comparisons between observers and imaging centers, as well as comparison with the published normal values.8–11 A complete short axis stack serves as the basis for quantification of biventricular volumes and function, as well as LV mass, with papillary muscles included in the blood volume as per convention. Delineation of contours in the basal slices can be challenging due to partial cuts through the structures, in which side-referencing with long axis views provides very helpful guidance.
MYOCARDIAL SCAR OR FIBROSIS BY LATE GADOLINIUM ENHANCEMENTGadolinium contrast-based imaging has unique ability to directly visualize the key extracellular pathophysiological substrates in the development of heart disease–myocardial scar/fibrosis.12 Late gadolinium enhancement exploits the differences in regional and temporal distribution of gadolinium-based contrast agents between different types of tissues such as healthy myocardium vs necrotic myocardium or scar tissue. Gadolinium is not imaged directly; imaging contrast is based on the alteration of the magnetic properties of tissue in the presence of the gadolinium, by “shortening” (acceleration) of the longitudinal relaxation within the tissues where gadolinium-based contrast agents accumulate. The LGE is best suited to visualization of well-demarcated regional changes, such as post-infarct myocardial scar13 (Figure 3). This phenomenon can be typically observed 10 min to 20min after administration of gadolinium-based contrast agents using T1-weighted inversion recovery gradient-echo sequences. The imaging contrast of black and white, ie, healthy (remote) myocardium and post-infarct scar, respectively, is achieved by “nulling” the healthy myocardium–an imaging preparation procedure, which allows identification of the time delay for inversion prepulse and suppression of the signal from healthy myocardium (therefore it appears black) to reveal the bright hyperenhancement of the scarred myocardium. In addition to post-infarction scar, LGE can also reveal nonischemic patterns of myocardial injury, such as patches of diffuse fibrosis or intramyocardial stria when sufficiently confluent and demarcated from the rest of the diseased myocardium (Figure 4). The histological correlate to this phenomenon, irrespective of the disease, is dense replacement fibrosis,12,14,15 which clinically represents a fixed irreversible injury. Its presence and extent is a relevant prognostic marker. Compared with other modalities able to delineate a fixed injury, such as SPECT (single-photon emission computed tomography), CMR with LGE is considerably more accurate in the detection of scar, owing to higher spatial resolution, whereby its presence, transmurality and extent provide information on prognosis and residual viability.13
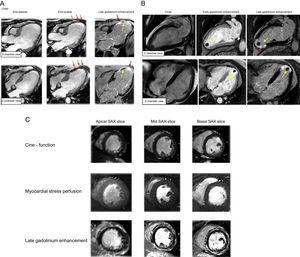
Side by side evaluation of cines and late gadolinium enhancement images. A: An example of ischemic cardiomyopathy. Cines in end-diastole and end-systole reveal thinned and akinetic myocardium in mid-apical anterior-anteroseptal-inferoseptal segments (orange arrows), corresponding with 100% transmural ischemic scar (red arrows). Intra-late gadolinium enhancement void of signal corresponds to the areas of microvascular obstruction (yellow arrows). B: An example of ischemic cardiomyopathy with apical thrombus adjacent to full-thickness myocardial scar. Imaging methods include cines, early and late gadolinium enhancement. C: An example of a patient presenting with angina-like symptoms. Absence of regional wall motion abnormalities and myocardial hypoperfusion excludes ischemic heart disease. Findings of non-ischemic like late gadolinium enhancement in epicardial-intramyocardial layers are consistent with myocarditis.
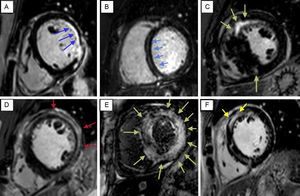
Typical patterns of late gadolinium enhancement supporting differentiation of various cardiomyopathies. A: Ischemic cardiomyopathy. B: Nonischemic cardiomyopathy. C: Hypertrophic cardiomyopathy. D: Myocarditis. E: Amyloidosis. F: Sarcoidosis. Late gadolinium enhancement allows to streamline this assessment to first address, whether this is an ischemic or non-ischemic cardiomyopathy; the typical ischemic late gadolinium enhancement pattern always affects the subendocardium (A shows a subendocardial ischemic hyperenhancement affecting mid-lateral and posterolateral segments of the left ventricle), with variable extension, from scars affecting less than 25% of myocardium thickness to transmural infarctions; once we have identified a particular late gadolinium enhancement as non-ischemic there are some morphologies that suggest different diseases. The presence of intramyocardial fibrosis, frequently affecting the midwall interventricular septum with longitudinal morphology (myocardial stria), suggests dilative cardiomyopathy (B) although epicardial, diffuse and focal patterns are also found. Hypertrophic cardiomyopathy (C) frequently presents with a patchy or confluent pattern, generally confined to segments with greatest wall thickening and at the right ventricle-left ventricle insertion points. Late gadolinium enhancement in myocarditis (D) has a nonischemic regional distribution, with focal subepicardial or intramyocardial scarring areas. Amiloid deposit within the myocardium significantly alters gadolinium kynetics, therefore resulting in images with a different appearence to the usual late gadolinium enhancement. Inability to null the myocardium in spite of the use of different inversion delays or focal, subendocardial enhancement strongly supports the diagnosis of amyloidosis. Focal scarring areas are often found in sarcoidosis (F), typically patchy, mid-wall or sub-epicardial, and frequently involving the anteroseptal and anterolateral walls of left ventricle and right ventricle.
The LGE sequences in clinical use are all based on a common principle of T1-weighted gradient echo acquisition, with differences in whether a single heartbeat (“single-beat”) or 2 heartbeats are used for image acquisition. The advantage of single-beats is the speed, allowing acquisition of a part or even whole of short axis stack in a single breath-hold, which will be appreciated by patients who find it difficult to tolerate being inside the scanner bore. The disadvantage is the commonly incomplete recovery of longitudinal magnetization (following the inversion prepulse needed to null the myocardium), with the addition of an extra angle at each next inversion–and as a consequence, the remote myocardium may be inadequately suppressed, appearing grey, and less strongly contrasting the bright scar tissue, especially in tachycardic patients. Whereas this would be unlikely to affect recognition of ischemic scar (high uptake of gadolinium, well regionalized), the observation of nonischemic types of LGE can be jeopardized. Acquisition over 2 heartbeats commonly provides an impeccable black-white contrast, but at the expense of longer breath-holds per single slice, as well as a lengthy acquisition of a stack of single slices. These 2 types of imaging may be either preselected or used consecutively, based on the pretest likelihood of ischemic vs nonischemic cardiomyopathy; the addition of 2-heartbeat LGE can always be considered if nonischemic scar seems likely or cannot be excluded with certainty following single-beat acquisitions.
The LGE by CMR provides a major distinction compared with other imaging modalities in the investigation of cardiovascular conditions. Initially conceived for its use in ischemic cardiomyopathy, it is capable of differentiating between dysfunctional–but viable myocardium that has the potential to recover from necrosis–and myocardial scar without any remaining viability, with near histology-like accuracy.14 The transmurality of the ischemic scar can be used to guide the decision on revascularization,13,16 as it provides information on residual viability and the probable benefit of revascularisation.13,17,18 The LGE also provides excellent visualization of complications of acute myocardial infarction, including LV thrombi or microvascular obstruction (no-reflow phenomenon, Figure 3). Both findings have implications for patient care, for anticoagulation therapy in the former and intensified antiremodeling treatment in the latter. Acquisition with the same type of sequences immediately after gadolinium contrast administration (early gadolinium enhancement) with a fixed long time-delay (> 400 ms) may further help to delineate these phenomena.
The LGE also provides information on the underlying etiology of myocardial dysfunction. Patterns of LGE support reliable differentiation between ischemic and nonischemic cardiomyopathies,19,20 as well as specific types of nonischemic cardiomyopathies.21 Indeed, it has been routinely used in the diagnosis of a variety of conditions such as myocarditis, hypertrophic and dilated cardiomyopathy and inflammatory or storage diseases, based on different enhancement patterns that can suggest the etiology of the cardiac dysfunction (Figure 4). The presence of nonischemic LGE and overall extent again relates to the burden of irreversible myocardial injury and is predictive of poor prognosis in these cardiomyopathies.15,22–25
PERFUSIONPharmacological stress perfusion under vasodilators (adenosine or regadenoson) is the noninvasive investigation of choice in suspected myocardial ischemia in patients with an intermediate pretest likelihood or in patients with known disease, in whom evidence of ischemia supports further intervention.26 A dynamic series of ultrafast T1-weighted gradient echo images is acquired at the time of rapid administration of a gadolinium-based contrast agent, allowing visualization of the first pass of gadolinium-based contrast agents in the myocardium (wash-in period), uncovering areas of compromised myocardial perfusion due to significant coronary artery stenosis. Increasingly, stress-perfusion is performed in isolation, ie, without additional rest-perfusion, since there is evidence that the diagnostic contribution of the latter is (alongside LGE images providing full information on myocardial fibrosis27) negligible. Rest-perfusion, if performed, has to follow the stress-perfusion, and not the other way around, because gadolinium accumulates in areas of scar with consequent enhancement (brightness), which obstructs the reading of hypoperfusion due to ischemia within that area. Several slices in different planes can be simultaneously acquired, including at least 3 short-axis slices and optionally long-axis planes in every heartbeat to evaluate all the 17 segments of the LV.1 Most clinical perfusion studies are evaluated visually and describe hypoperfusion in terms of localization (corresponding to a perfusing territory of a coronary artery), the number of affected segments, and transmurality. Semiquantitative methods and absolute quantification of myocardial flow remain under investigation.28
Myocardial stress perfusion is a highly validated and accurate noninvasive method to confirm the presence of significant myocardial ischemia.29 This technique can be used to assess myocardial perfusion reserve and can reliably detect coronary artery disease with invasive angiography as the gold standard30 (sensitivity, 88%; specificity, 90%; diagnostic accuracy 89%) even in the presence of 1-vessel disease. The performance of perfusion CMR compared with SPECT has been evaluated in several real-world prospective high-quality trials,29,31,32 involving multivendor technology and populations with different risk profiles and presence of coronary disease. Despite this variability, they showed similar results, which were in agreement with previous studies, with CMR providing a better performance than SPECT, mainly due to higher sensitivity, with similar or lower specificity. These results were consistent across the study population, including 1-, 2- or 3- vessel disease, and were independent of the cutoff for significant stenosis per angiography. Unfortunately, functional assessment of coronary stenosis was not included in these trials, anatomic evaluation with angiography being an imperfect gold standard, which could be the source of false-positive or negative results of functional tests. Cardiovascular magnetic resonance also supports comparison with positron emission tomography regarding the calculation of myocardial perfusion reserve,33 although both techniques have a weak correlation and require further refinement.
As an alternative to adenosine stress perfusion, dobutamine stress MRI is a feasible choice to assess myocardial ischemia, by detecting stress-induced wall motion abnormalities. Indeed, the diagnostic performance of dobutamine stress MRI is superior to that of adenosine stress,34 the latter being underpowered to detect wall motion abnormalities (only in the presence of perfusion defects > 75%). Adenosine perfusion has excellent sensitivity at the expense of lower specificity. However, dobutamine stress MRI involves a higher risk of adverse events, including ventricular arrhythmias and hypotension, and its performance requires the physical presence of a highly skilled team and advanced life support material.
Most importantly, both stress modalities have shown an important prognostic value as they are able to discriminate patients at low and high risk of developing cardiac events, the 3-year event-free survival after a negative adenosine and dobutamine stress MRI being > 99%.35 Moreover, a negative result of any of these stress tests provides important prognostic information over classical clinical risk factors. However, the important question is not only if there is or is not ischemia, but the amount of ischemic myocardium; moreover, the “ischemic burden”36 plays a central role in cardiac outcomes. Different thresholds derived from different imaging techniques have been used to assess prognosis, but a recent review suggests that 4 or more of 32 stress perfusion defects or 3 or more of 16 dobutamine-induced dysfunctional segments confer an annual risk of cardiac death or myocardial infarction of approximately a 5%.37
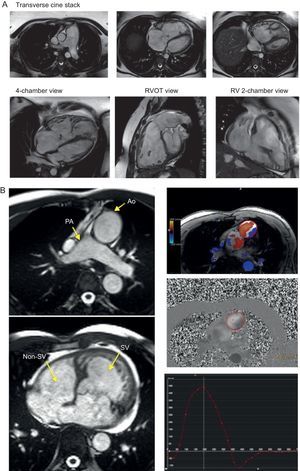
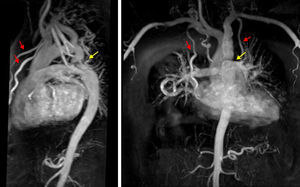
SEQUENCES SUPPORTING SPECIAL PROTOCOLSRight Heart AssessmentAdditional views of structures of the right heart are afforded in special indications, such as adults with pediatric or congenital heart disease or pulmonary hypertension, and include the additional stack in continuous transverse slices, right ventricular outflow tract and right ventricular 2-chamber view (Figure 5). Although transverse stack is the mainstay acquisition mode in imaging of adults with congenital heart disease, 3-dimensional volume acquisitions of still balanced images acquired in end-diastole (without the need for contrast agents) also helps to support exact visualization of the morphology and anatomical relations between the great vessels and the heart. These are commonly preferred (especially in the pediatric population) over classical contrast-enhanced MRI angiographies, because they allow inspection of relationships between intrathroracic vessels and structures, as opposed to an outline of vessels by chasing an arterial phase of contrast opacification (Figure 6). Although the significance of cardiac shunts can be evaluated by comparison of ventricular stroke volumes, the Qp:Qs ratio can be accurately measured by through-plane flow measurements of the great vessels using phase-contrast velocity mapping sequence. This sequence uses the phase difference magnetization between mobile and immobile spins to determine the flow profile over a cardiac cycle, as well as spins moving with different speeds. This technique also allows measurement of valvular stenosis and regurgitations, in which its use in aortic and pulmonary valve disease is more consistent and has been better validated than in mitral and tricuspid disease.
Assessment of the right heart. Specific views for more detailed assessment of right cardiac chambers can be planned from the basic cardiac views and are of the utmost importance in conditions affecting the right ventricle such as congenital heart disease or pulmonary hypertension. A: An example of pulmonary hypertension; a transverse cine stack (an axial plane including the whole right ventricle and pulmonary artery) allows us to easily visualize right apical structures and the transition to right ventricular outflow tract and pulmonary artery; 4-chamber, right ventricular outflow tract view and right ventricular 2-chamber view are especially useful in assessment of regional wall motion abnormalities of right ventricle free wall in arrhythmogenic right ventricular cardiomyopathy and congenital heart disease. B: An example of congenitally corrected transposition of the great arteries. Phase-contrast images of aortic and pulmonary flow can be quickly obtained, allowing the calculation of systolic volumes, aortic/pulmonary regurgitation and Qp:Qs to ascertain the presence of a shunt. Ao, aorta; PA, pulmonary artery; RV, right ventricular; RVOT, right ventricular outflow tract; SV, systolic volume.
A transverse stack of contiguous balanced slices is the first step to detailed visualization. Once the structure of interest has been identified and its regional relationships clarified, tissue characterization may add information. Conventionally, T1 and T2 spin echo sequences with fat suppression may be used, as well as perfusion, early and LGE (vascularity and fibrosis). T1 and T2 black-blood sequences have many disadvantages, including slow blood flow artefacts, due to oblique blood flow and inadequate blood signal suppression, as well as motion artefacts due to long acquisition times. In terms of additional information, the yield of T1 and T2 sequences to tissue characterization—compared with cines, perfusion and LGE—in cardiac masses tends to be minimal and limits the use of these sequences based on traditional approaches.
Modern Tissue Quantitative Techniques (Tissue Mapping)In patients receiving blood transfusions, a single breath-hold T2* acquisition is able to inform the myocardial iron overload and relate to the risk of adverse events, as the first successful quantitative myocardial tissue imaging method. Emerging evidence on myocardial tissue relaxation quantification by T1 and T2 mapping38–40 holds the promise of conquering diffuse myocardial involvement, such as myocardial fibrosis and inflammation, which is of strong relevance to the paucity of treatment options in nonischemic cardiomyopathies.41 In a considerable number of these conditions, heart muscle is affected diffusely, as well as in a progressive continuum of myocardial involvement. These imaging methods allow quantification of magnetization recovery or decay, respectively, within myocardial tissue, the rates of which are altered in disease. T1 mapping measurements can provide clinically meaningful indices, including native T1, post-contrast T1 and a hematocrit-corrected partition coefficient, the extracellular volume fraction. To calculate the latter, blood sampling for hematocrit needs to be performed at the time of the scan.42 These measurements have been reported using a variety of sequences, which have their strengths and limitations, by way of acquisition and post-processing, in terms of T1 accuracy and precision of measurements, as well as diagnostic accuracy in a variety of cardiac conditions. Thus far, the evidence reveals that T1 mapping indices reflect abnormal myocardium in a variety of cardiac conditions42 and relate to indices of myocardial systolic and diastolic performance.43,44 Cumulatively, evidence suggests that T1 mapping indices could be useful in the detection of diffuse myocardial disease, complementing LGE as a tool for regional myocardial disease.
CONCLUSIONCardiac magnetic resonance offers a comprehensive morphologic and functional evaluation of the heart. A focused approach to answer critical clinical questions can be accomplished in most cases with a basic framework of left ventricular function and LGE, and addition of perfusion protocol, which allows a rapid evaluation and will facilitate generalization of this technique outside expert, usually research-focused centers. Its tissue characterization capacity offers additional and important information in cardiomyopathies, with a promising future of new mapping techniques.
CONFLICTS OF INTERESTV.O. Puntmann holds a consultancy agreement with Circle Cardiovascular Imaging Ltd. to provide educational material.
The authors would like to acknowledge the insights and advice provided by Prof. Eike Nagel (Department of Cardiovascular Imaging, Goethe University, Frankfurt) and Dr. Eltjo Haselhof (Philips Healthcare) for their efforts to facilitate concise imaging schemes in contemporary clinical CMR practice.