Transcatheter aortic valve replacement is an established therapy for patients with symptomatic severe aortic stenosis and contraindications or high risk for surgery. Advances in prostheses and delivery system designs and continuous advances in multimodality imaging, particularly the 3-dimensional techniques, have led to improved outcomes with significant reductions in the incidence of frequent complications such as paravalvular aortic regurgitation. In addition, data on prosthesis durability are accumulating. Multimodality imaging plays a central role in the selection of patients who are candidates for transcatheter aortic valve replacement, procedure planning and guidance, and follow-up of prosthesis function. The strengths and limitations of each imaging technique for transcatheter aortic valve replacement will be discussed in this update article.
Keywords
Transcatheter aortic valve replacement (TAVR) has become a safe and feasible alternative treatment for patients with severe aortic stenosis (AS) who have contraindications or are at high risk for surgical aortic valve (AV) replacement. In terms of survival and improvement in clinical symptoms, large randomized clinical trials have proven TAVR to be superior to medical therapy (and balloon valvuloplasty) in patients deemed inoperable1,2 and noninferior to surgical AV replacement in patients with high operative risk.3,4 These results encouraged the rapid implementation of TAVR in current practice with more than 200 000 patients treated worldwide.5 Patient selection, accurate sizing of the prosthesis, and procedural planning require the use of several imaging modalities to optimize results and minimize complications such as paravalvular regurgitation (PVAR), pacemaker implantation, vascular injury, or annulus rupture. Procedural guidance is mainly performed under fluoroscopy assistance and, still, in many laboratories, with the help of transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE). The steep learning curve of the procedure and the low number of periprocedural complications in high-volume centers have allowed less invasive TAVR by implanting the device under conscious sedation. Therefore, the need for TEE during the procedure has recently been questioned. In addition, prosthesis durability is an important factor to eventually expand this procedure to patients with low-intermediate operative risk. Five-year follow-up data from the PARTNER trial6,7 showed no structural degeneration of the balloon expandable prosthesis with stable transvalvular gradients and aortic valve areas (AVA). However, the use of high spatial resolution imaging techniques such as multidetector row computed tomography (MDCT) have raised concern due to the presence of thickening and restriction of the prosthetic leaflets, suggesting subclinical thrombosis that could not be appreciated with echocardiography.8 The present update article summarizes the role of multimodality imaging for preprocedural planning (patient selection, device sizing, and procedural access), procedural guidance and follow-up, highlighting the pros and cons of each imaging modality.
PREPROCEDURAL PLANNINGAccurate assessment of AS severity, AV and root anatomy and geometry, and evaluation of the feasibility of peripheral vascular access are 3 key steps during planning of TAVR.
Aortic Stenosis SeverityDoppler TTE is the imaging technique of choice to assess AS severity.9,10 It provides key insights into AV anatomy, degree of calcification, hemodynamic consequences of AS (left ventricular [LV] size, wall thickness and function, pulmonary arterial pressure), concomitant valve disease, and aortic pathology. Severe AS is defined as aortic jet velocity >4 m/s, mean transvalvular pressure gradient >40mmHg, and calculated AVA <1.0cm2.9,10 There are situations, however, in which these parameters are not congruent, challenging the diagnosis of severe AS and patient management.
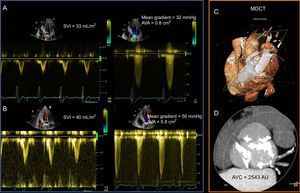
When severe AS coexists with reduced LV systolic function, the flow derived indices may overestimate the severity of AS. This condition is termed classical low-flow low-gradient AS and is characterized by reduced left ventricle ejection fraction (LVEF), an AVA <1.0cm2, aortic velocity <4 m/s, mean gradient <40mmHg, and stroke volume index <35mL/m2.10,11 In this subgroup of patients, differentiation between true severe AS and pseudosevere AS has important therapeutic implications.12,13 Using low dose dobutamine stress echocardiography, the contractile reserve of the LV is increased, leading to an increase in LV stroke volume (flow).11 In a true severe AS, the increase in flow is associated with an increase in transvalvular gradients while the AVA remains <1.0cm2. In contrast, in pseudosevere AS, the increase in LV contractility and flow results in an increase in AVA >1.0cm2 while the transvalvular gradients remain low (Figure 1). However, 30% to 40% of patients with classical low-flow low-gradient severe AS do not show contractile reserve during low dose dobutamine stress echocardiography.13 In this specific group of patients, the use of computed tomography and the assessment of AV calcification burden may help to estimate the severity of AS (Figure 1).14,15 Cutoff values of AV calcification = 1274 AU in women and = 2065 AU in men were more frequently associated with severe AS.14
Low-dose dobutamine stress echocardiography and aortic valve calcification assessment with multidetector row computed tomography in a patient with low-flow low-gradient severe aortic stenosis with reduced left ventricular systolic function. A: Baseline echocardiographic assessment revealed discrepant indices of aortic stenosis severity. The mean gradient was 32mmHg and aortic valve area was 0.8cm2. Stroke volume index was 33mL/m2. B: Low-dose dobutamine stress echocardiography resulted in an increase of the mean gradient to 50mmHg, the aortic valve area remained unchanged and the stroke volume index increased by 21%. This indicates that the patient had classical low-flow low-gradient severe aortic stenosis and the presence of flow reserve (stroke volume index increased > 20%). C: Volume rendered cardiac multidetector row computed tomography with a plane across the aortic annulus. D: Aortic valve calcification load, using the Agatston method, was measured 2543 AU, indicating severe aortic stenosis (cutoffs for severe aortic stenosis = 2,065 AU in men and = 1,274 AU in women14. AVA, aortic valve area; AVC, aortic valve calcification; MDCT, multi-detector row computed tomography; SVI, stroke volume index.
Patients with paradoxical low-flow low-gradient severe AS present with preserved LVEF, AVA <1.0cm2, mean gradient <40mmHg, and LV stroke volume index <35mL/m2.9,10 In this subgroup of patients, the low-flow condition is determined by the small LV cavity due to severe LV hypertrophy. The management of these patients remains challenging. Clavel et al16 compared the outcome of 187 patients with paradoxical low-flow low-gradient severe AS with 187 patients with severe AS and high gradient (matched according to AVA) and with 187 patients with moderate AS (matched according to mean transvalvular gradient) and showed that patients with paradoxical low-flow low-gradient severe AS have reduced overall survival (1-year, 89±2%; 5-year, 64±4%) compared with patients with high gradient severe AS (1-year, 96±1%; 5-year, 82±3%) or moderate AS (1-year, 96±1%; 5-year, 81±3%). Moreover, AV replacement was significantly associated with improved survival in patients with paradoxical low-flow low-gradient severe AS, but not in the moderate AS group.16 Of note, the study population was relatively heterogeneous with a significant proportion of patients being asymptomatic and with heterogeneous management (80% of patients with severe AS and high gradient underwent AV replacement compared with 56% in the group of paradoxical low-flow low-gradient and 40% in moderate AS). In contrast, Jander et al17 demonstrated that patients with asymptomatic severe AS, low gradient and preserved LV ejection fraction (low stroke volume index <35mL/m2 was present in 51%) had similar outcomes to those of patients with moderate AS (major cardiovascular events 14.8±1.0% vs 14.1±1.5%, respectively; P=.59).
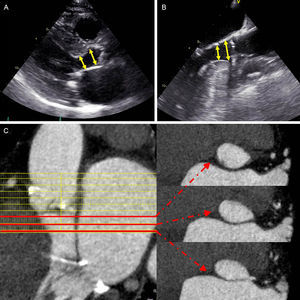
According to current guidelines, the finding of paradoxical low-flow low-gradient AS should be approached stepwise.9 Any source of error in measured parameters of the continuity equation used for AVA calculation should be addressed first. Left ventricular outflow tract (LVOT) cross-sectional area is one of the key parameters. With 2-dimensional echocardiography, LVOT cross-sectional area is traditionally derived by measuring the midsystolic sagittal LVOT diameter in the parasternal long-axis view, assuming a circular geometry. However, a sigmoid septal basal hypertrophy characteristic of elderly patients may challenge the accuracy of this method since the LVOT may become elliptical (Figure 2).18,19 By measuring the planimetric area of the LVOT with a 3-dimensional (3D) imaging technique such as MDCT and introducing the value into the continuity equation, it has been demonstrated that 33% of the low-gradient severe AS patients with preserved LVEF could be reclassified into moderate AS.20 If body surface area is small, correction for body surface area is necessary, with an AVA index <0.6cm2/m2 indicating severe AS. A severely increased global hemodynamic afterload (ie, valvulo-arterial impedance) should be also excluded. Furthermore, particular attention should be paid to accurately determine the LV stroke volume, preferably by confronting measurements from other independent methods (two-dimensional or 3D volumetric methods by means of echocardiography, cardiac magnetic resonance [CMR], or MDCT). Low dose dobutamine stress echocardiography can provide additional information about the actual severity of the AS and can predict the risk of adverse events, but the safety of dobutamine stress echocardiography in patients with pronounced LV concentric remodeling and small LV cavities has yet to be established.21 In addition, evaluation of the degree of AV calcification by computed tomography may be of help in this group of patients.14,15
Assessment of left ventricular outflow tract with transthoracic (A) and transesophageal (B) echocardiography and multidetector row computed tomography (C). On 2-dimensional transthoracic and transesophageal echocardiography the measurement of the left ventricular outflow tract may vary significantly (arrows), particularly in patients with sigmoid septum, which has important implications on aortic valve area calculation. On multidetector row computed tomography, red lines depict left ventricular outflow tract areas at 3 different levels, showing the change in area and ellipsicity of the left ventricular outflow tract.
In contrast to surgical AV replacement, where surgeons can directly determine the optimal prosthesis size and visualize the adaptation of the prosthesis to the aortic root, in TAVR appropriate prosthesis selection relies mostly on preprocedural imaging. Too small prostheses increase the risk of significant PVAR and prosthesis migration, while oversized prostheses may lead to incomplete deployment, potentially resulting in both valvular and paravalvular regurgitation or even catastrophic aortic annulus rupture.22,23
Three-dimensional imaging techniques (3D echocardiography, MDCT, CMR) are currently the preferred tools to assess the aortic annulus size. Sagittal aortic annulus diameter, normally measured with 2-dimensional echocardiography, tends to underestimate the true aortic annulus size.24 In contrast, studies using 3D TEE or MDCT have shown that selection of prosthesis size based on these imaging modalities is associated with a lower incidence of significant PVAR.25–27 These 3D imaging techniques permit the measurement of the aortic annulus area and perimeter using direct planimetry and diameters derived from the area and the perimeter. Most manufacturers have also included these measurements in the prosthesis size charts, allowing the standardization of the prosthesis selection.
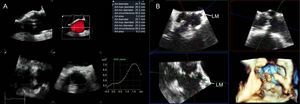
The MDCT provides high spatial resolution images of the aortic annulus and aortic root. This imaging technique has become key in TAVR due to its low invasiveness and comprehensive evaluation of candidates for TAVR, including assessment of aortic annulus, burden of AV and root calcification, and peripheral arteries anatomy (Figure 3). In addition, MDCT permits planning of the C-arm projections needed for AV balloon dilatation and prosthesis deployment, reducing the need for repeated angiographies during the procedure.28,29 However, in patients with associated impaired renal function, the use of MDCT should be tailored to reduce the risk of periprocedural acute kidney injury. Three-dimensional TEE has also been shown to be of value to size the aortic annulus, aortic root dimensions, AV calcification burden, and height of coronary ostia relative to the aortic annulus (Figure 4).30 This imaging modality is, however, relatively uncomfortable for patients and the acoustic shadowing caused by the aortic cusp calcifications may impact on the spatial resolution of the images and on the accuracy of the measurements. Cardiac magnetic resonance permits 3D analysis of the aortic annulus and root anatomy similarly to MDCT. However, this imaging technique is less available and is not feasible in patients with non-CMR compatible implanted devices. These 3D imaging modalities have been compared in several studies, showing similar accuracy in sizing the aortic annulus.24,31,32 Of note, data acquisition should preferably be performed with electrocardiogram gating to obtain the systolic and diastolic dimensions of the aortic annulus. A recent study by Murphy et al33 including 507 patients with severe AS who underwent ECG-gated MDCT showed significant changes in aortic annulus area and perimeter between systole and diastole (8.23% and 3.36%, respectively). The implications of these findings are relevant since the use of the diastolic measurement would have resulted in a change of the prosthesis size (undersizing) in 50% of the patients. Therefore, assessment of systolic and diastolic measurements is recommended.34
The role of multidetector row computed tomography in preprocedural assessment. A: Double oblique transverse view of severely calcified tricuspid aortic valve. B: Planimetry of the aortic annulus. C: Measurement of the distance between the left main coronary artery and the aortic annulus (arrow). D: Computed tomography aortography reveals severely calcified aorta, particularly in the aortic arch and in the descendent part. Calcifications are present in both iliofemoral arteries as well.
Three-dimensional transesophageal echocardiography in transcatheter aortic valve replacement planning. A: Automated analysis of the aortic root (AVQ software, GE, Horten, Norway) allows quick alignment of the orthogonal planes across the aortic annulus and accurate sizing. B: Multiplanar 3-dimensional reconstruction of the aortic root to measure the distance between the left main coronary artery and the aortic annulus (arrows). At the same time the presence of bulky calcified cusps that may obstruct the coronary ostia can be appreciated, particularly in the 3-dimensional reconstruction. LM, left main coronary artery; SAX, short axis.
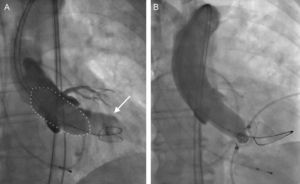
During the procedure, aortic annulus can be also measured with supra-aortic angiography during balloon aortic valvuloplasty (Figure 5). Several studies have shown the accuracy of this methodology to size the prosthesis.35,36 During the balloon valvuloplasty, the presence of residual PVAR on angiography indicates an undersized balloon.35 Other authors have proposed measurement of the balloon with sterile callipers during inflation at 2 atms and, during full volume balloon inflation at the level of the valve, any additional increase in the intraballoon pressure of more than 2 atms will indicate that the diameter of the balloon is equal to or larger than the aortic annulus.36
Supra-aortic angiography during balloon aortic valvuloplasty for prosthesis size selection. A: A 23-mm balloon (dotted line) was chosen for a preparatory balloon aortic valvuloplasty according to the 2-dimensional transesophageal echocardiography data on the aortic annulus size. Concurrent supra-aortic angiography, showing contrast regurgitation into the left ventricle (arrow), indicated annulus size underestimation by 2-dimensional transesophageal echocardiography and resulted in the selection of a larger prosthesis. B: Absence of contrast regurgitation into the left ventricle during balloon aortic valvuloplasty with a 23-mm balloon confirmed correct annular sizing based on preinterventional 2-dimensional transesophageal echocardiography. Reproduced with permission from Patsalis et al.35
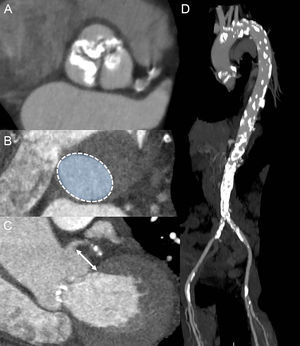
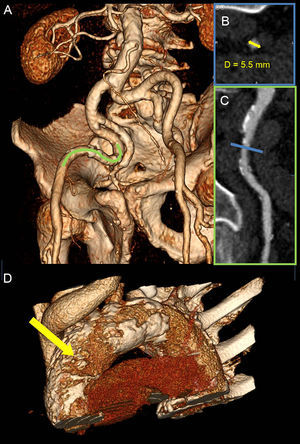
One of the key aspects of preprocedural planning in TAVR is to choose the optimal access route. Potential TAVR access sites are transfemoral (TF), transapical, transaortic, transsubclavian, transaxillary, and transcarotid. The predominant approach worldwide is TF, since it is the least invasive technique and the most familiar to interventional cardiologists. According to the data from TAVR registries, the TF approach is chosen in 71% to 75% of procedures in Europe37,38 and in 56% in the United States.39 The suitability of the TF approach is predominately evaluated with angiographic assessment of the iliofemoral anatomy during coronary angiography. However, MDCT has shown better characterization of iliofemoral arteries and aortic size, tortuosity, degree of calcifications, and plaque burden (Figure 6). Moreover, detailed vascular anatomy can be clearly visualized with 3D volume rendered and multiplanar reconstructions. For currently available TAVR delivery catheters, a 6.0 mm to 6.5mm threshold for minimal luminal vessel diameter of the femoral artery is considered to be acceptable.40
Assessment of transcatheter aortic valve replacement access with multidetector row computed tomography. A: Severely tortuous bilateral iliofemoral arteries, visualized with 3-dimensional volume rendering. B: The cross sectional lumen of the narrowest part of the vessel (blue line in C) was assessed. The smallest diameter was 5.5 mm (yellow arrow), precluding a safe transfemoral approach for the transcatheter aortic valve replacement. C: A segment of the right external iliac artery (green line in A) was more closely studied in a multiplanar reconstruction plane, outlining high atherosclerotic burden with multiple plaques. D: Severely calcified thoracic aorta–porcelain aorta, particularly in the anterolateral portion of the ascending aorta, corresponding to the landing zone for transaortic approach (yellow arrow).
Traditionally, the transapical approach is preferred for patients whose peripheral vasculature is not suitable for TF. However, transapical access is the most invasive technique and might be contraindicated in patients with certain comorbidities or high frailty indexes (severe pulmonary disease, chest wall deformity, very poor LV function, intracavitary thrombus). Alternatively, the transaortic approach has gained popularity due to the simplicity of the procedure and superior results compared with transapical access in terms of survival.41,42 The MDCT analysis of the ascending aorta is essential in selecting patients for transaortic TAVR. The anterolateral portion of the ascending aorta 5 cm to 7cm above the aortic annulus, where cannulation of the aorta takes place (known as the transaortic landing zone), should be free of calcium (Figure 6). Bapat et al43 have shown that the transaortic approach is feasible in patients with severe aortic calcifications (porcelain aorta) since the transaortic landing zone is frequently spared. Moreover, MDCT permits evaluation of the spatial relationships between sternum and major vessels in the thorax. This is particularly important in patients with previous coronary artery bypass surgery, in which close proximity of the aforementioned structures or high proximal venous graft anastomoses affect the preferred transaortic access route (eg, opting for mini right thoracotomy instead of mini J sternotomy).42
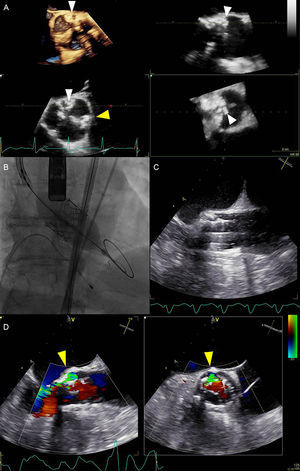
IMAGING DURING TRANSCATHETER HEART VALVE IMPLANTATIONProcedural guidance during TAVR has been traditionally performed under fluoroscopy and angiography with the support of TEE (Figure 7).44 This approach is still advocated by the EAE/ASE (European Association of Echocardiography/American Society of Echocardiography) recommendations.45 However, the current generation of TAVR devices with smaller delivery systems has increased the feasibility of the TF approach and reduced procedural timings and invasiveness (similar to balloon valvuloplasty), thus placing in doubt the need for general anesthesia. Indeed, some large European TAVR centers have demonstrated excellent feasibility and safety of a simplified TF approach, performed using monitored anesthesia care (defined as cardiovascular and respiratory monitoring of the patient by a qualified anesthesiologist who may or may not be administering concomitant sedation46) or local anesthesia only.47–49
Multimodality imaging during transcatheter aortic valve replacement. A: Periprocedural 3-dimensional transesophageal echocardiography revealed severely calcified tricuspid aortic valve. Particularly prominent calcifications were at the level of left- and noncoronary cusps commissure (white arrowheads) and at the level of left- and right-coronary cusps commissure (yellow arrowhead). B: Balloon expandable transcatheter valve deployment, guided by fluoroscopy. C: Concurrent real-time 2-dimensional transesophageal echocardiography image of the valve deployment. D: Paravalvular aortic regurgitation visualized with colour Doppler biplane echocardiography (yellow arrowheads). Paravalvular aortic regurgitation originated at the level of highest annular calcification burden. The circumference of the paravalvular aortic regurgitation was 20% of the prosthesis frame (short axis view on the right side), suggesting moderate paravalvular aortic regurgitation according to Valve Academic Research Consortium-2 criteria.44
However, TEE, especially real-time 3D TEE, offers an incremental value over fluoroscopic and angiographic guidance in TAVR: it supports crossing severely calcified native AV, significantly reduces radiation exposure and the use of nephrotoxic iodine contrast,50 and allows detection of life-threatening complications at an early stage. Aortic annulus rupture, perforation of the myocardium with subsequent pericardial hemorrhage, coronary ostia occlusion resulting in myocardial ischemia, aortic perforation or dissection, prosthesis malpositioning or dislodgement, and valvular or paravalvular leaks are complications that can be immediately detected by TEE and influence decision-making (Figure 7).
Although currently available prostheses have been associated with a lower incidence of significant aortic regurgitation after TAVR,51,52 this complication remains a cause of concern since it has been associated with poor prognosis.7,53 Evaluating the presence and severity of aortic regurgitation should include assessment of both central and paravalvular components, with a combined measurement of “total” aortic regurgitation, reflecting the total volume load imposed on the LV. The methods used in native valve regurgitation (qualitative assessment of color flow Doppler, vena contracta, pressure half-time on the continuous-wave Doppler recordings) are limited in the setting of paravalvular jets, which are frequently multiple, eccentric, and irregular in shape. Moreover, certain portions of the prosthesis ring and LVOT may be difficult to image due to acoustic shadowing. The EAE/ASE guidelines for evaluation of the prosthetic valves propose the proportion of the circumference of the sewing ring, occupied by the jets, as an alternative semiquantitative measure of PVAR severity: <10% of the sewing ring suggests mild, 10% to 20% moderate, and >20% suggests severe PVAR.54 The Valve Academic Research Consortium-2 has slightly modified these cutoff values in the TAVR setting: mild, moderate, and severe PVAR are defined by <10%, 10% to 29%, and = 30% of the circumference of the prosthesis frame, respectively (Figure 7).44 Regurgitant volume calculation can also be helpful in the TAVR setting. This method relies on comparison of stroke volumes across the AV and another nonregurgitant valve (either mitral or pulmonary). The former can be obtained by subtracting the LV end-systolic volume from the end-diastolic volume or (more commonly) by employing the continuity equation and calculating the stroke volume across the AV. The difference between the stroke volume across AV and the nonregurgitant valve represents the estimate of total AV regurgitant volume. Secondary indices, such as diastolic flow reversal in descending aorta, may provide additional help in assessing the severity of PVAR after TAVR.
Another alternative periprocedural imaging method is transnasal TEE.23,45 Smaller transnasal probes allow prolonged monitoring without general anesthesia. However, image quality is lower compared with conventional TEE and transnasal probes do not have 3D capabilities. Some centers have adapted intracardiac echo for TAVR guidance.55 The intracardiac echo probe is advanced through the femoral vein into the right atrium, where it brings a close-up view of the aortic root. In addition to obviating the need for general anesthesia, intracardiac echo allows uninterrupted monitoring in TAVR (no fluoroscopic interference) and more feasible Doppler-based assessment of pulmonary artery pressures.55 Intracardiac echo technology is quickly developing, also allowing live 3D imaging (though with a limited 22° to 90° volume). However, the widespread use of intracardiac echo in TAVR is limited by the need for high expertise, lower image quality in comparison with TEE (especially 3D), possible interference with the pacemaker lead, and particularly its high cost.
LONG-TERM FOLLOW-UPAfter TAVR, TTE remains the imaging technique of choice to evaluate procedural results, the durability of the prosthesis, and changes in LV dimensions and function. Post-discharge clinical, electrocardiographic, and TTE evaluations at 30 days after TAVR are mandatory.23,44 Further follow-up recommendations suggest TTE evaluation at 6 months and 1 year following implantation and yearly thereafter.44 The frequency of follow-up evaluations should be increased if there is any change in clinical status or worsening of echocardiographic findings. However, as experience with TAVR grows, the frequency of TTE assessment may likely decline toward that of surgical AV replacement with proposed annual check-ups 5 years after valve implantation.54
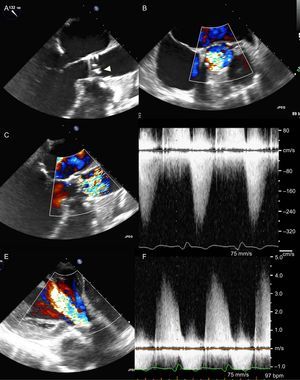
In terms of durability of the implanted prosthesis, valve position, the morphology of the prosthetic leaflets, and indices of valve stenosis and regurgitation should be evaluated with echocardiography (Figure 8). When calculating the effective orifice area or another index of valve opening that employs the ratio of pre- to post-valvular velocities (eg, Doppler velocity index), it is essential to record the prevalvular velocity (and LVOT cross-sectional area) immediately proximal to the stent of the implanted prosthesis. Due to the flow acceleration within the stent, measuring velocities even proximal to the valve cusps results in an overestimation of effective orifice area or AVA.23,44,45 Clavel et al56 reported slightly superior hemodynamic performance of transcatheter prostheses compared with the surgical bioprostheses. Fifty patients who underwent TAVR were matched 1:1 for sex, aortic annulus diameter, LV ejection fraction, body surface area, and body mass index with 2 groups of 50 patients that underwent surgical AV replacement with a stented or stentless valve prosthesis. Mean transvalvular gradients at 6 months to 12 months after the procedure were significantly lower in the TAVR group (10±4mmHg) compared with the surgical AV replacement group with a stented frame prosthesis (13±5mmHg) and were nonsignificantly different to the surgical AV replacement group with a stentless valve (9±4mmHg).56 Better hemodynamic results of the transcatheter valves were attributed to the thinner stent frameworks. In addition, the 5-year follow-up results of the PARTNER trial7 show stable hemodynamic performance of the transcatheter and surgical prostheses without signs of valve degeneration. However, in a retrospective analysis of 4266 patients who underwent TAVR in 12 different centers worldwide, Latib et al57 showed an incidence of 0.61% of transcatheter valve thrombosis after a median follow-up of 6 years. Of the 26 patients with suspected valve thrombosis, 92% presented with raised mean transvalvular gradients>20mmHg and 65% had exertional dyspnea. Anticoagulation resulted in a significant decrease of transvalvular gradients in all medically treated patients.57 However, recent studies using 4-dimensional MDCT have suggested that transcatheter valve thrombosis may be more frequent. Leetmaa et al58 reported an incidence of 4% in a cohort of 140 patients who underwent MDCT 1 month to 3 months after TAVR. Transcatheter valve thrombosis was defined by the presence of low-attenuation masses attached to valve cusps or a diffuse thickening of = 1 valve cusps. Anticoagulation treatment was successful, leading to a complete resolution of thrombi on a follow-up MDCT.58 These MDCT findings may not be accompanied by changes in symptoms or changes in valve hemodynamics as assessed with TTE, suggesting that MDCT may detect valve thrombosis at an earlier stage. Makkar et al8 reported reduced bioprosthesis leaflet motion, detected on 4-dimensional volume-rendered MDCT scans, in 40% (22 of 55 patients) in the PORTICO IDE (Portico Re-sheathable Transcatheter Aortic Valve System U.S. Investigational Device Exemption) trial and in 13% (17 of 132 patients) in 2 registries of aortic transcatheter and surgical bioprostheses in the United States and Denmark. Restoration of leaflet motion was noted in all 11 patients who started warfarin anticoagulation after MDCT findings and in only 1 of 10 patients who did not.8 Of note, again no echocardiographic indices of valve dysfunction were noted. These findings indicate the need for prospective, well-designed, and adequately powered studies that will provide relevant answers about the clinical significance of these findings (both in terms of neurological outcome and prosthesis durability), the optimal antithrombotic treatment after TAVR, as well as the imaging approach in the long-term follow-up.
Prosthesis degeneration 4 years after transcatheter valve replacement. A: Transesophageal echocardiography shows focally thickened and calcified prosthesis leaflets (arrowhead). B: Color Doppler image in the midesophageal short-axis view of the aortic valve reveals turbulent antegrade flow in a limited cross-sectional area. C: Color Doppler of the long-axis view confirms high turbulence downstream the prosthesis, implying severe prosthetic valve stenosis. D: High gradients obtained with continuous wave Doppler confirm significant prosthesis stenosis. E: Color Doppler transgastric view shows severe aortic regurgitation. F: High density and steep downsloping of the continuous wave Doppler recordings of the regurgitant flow confirm severe aortic regurgitation.
Changes in PVAR grade over time should also be evaluated at follow-up. In addition to TTE and TEE, CMR may be employed to assess the severity of PVAR. Cardiac magnetic resonance phase-velocity mapping of the blood flow in the ascendant aorta allows independent estimation of the AV regurgitant volume and regurgitant fraction.59 Sherif et al60 have shown that quantitative measurements of aortic regurgitation by CMR is superior to semiquantitative echocardiographic assessment with color flow Doppler imaging and that the latter may underestimate the degree of PVAR after TAVR.
Another adverse outcome after TAVR is infective endocarditis. Results from a large multicenter study report an incidence of 0.50% of infective endocarditis at 1 year after TAVR.61 However, the outcome is devastating, with 47% and 66% mortality during the index hospitalization and at 1 year follow up, respectively.61 Transthoracic echocardiography and, particularly with prosthetic valves, TEE are the first choice imaging techniques in the diagnostic workup of suspected infective endocarditis, helping to reveal the presence of vegetations, abscesses, pseudoaneurysms, their hemodynamic consequences (usually severe valvular or paravalvular aortic regurgitation), and possible involvement of other valves (eg, extension to anterior mitral leaflet) and to evaluate LV function. Importantly, infective endocarditis should always be suspected in patients with new periprosthetic regurgitation until proven otherwise.62 Real-time 3D TEE is of incremental value for the analysis of vegetation morphology and size and may improve prediction of embolic risk.63 The MDCT can be used to detect abscesses/pseudoaneurysms with a diagnostic accuracy similar to TEE and is possibly superior in assessing the extent of perivalvular infective endocarditis.64 In addition, nuclear imaging techniques, particularly radiolabeled white blood cell single-photon emission computed tomography/computed tomography and 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging, are evolving as important supplementary methods for patients with suspected infective endocarditis. The main added value of these techniques is the reduction in the rate of misdiagnosed infective endocarditis, classified in the “possible infective endocarditis” category using the Duke criteria, as well as the detection of peripheral embolic events.65
CONCLUSIONSThe TAVR is an established therapy for patients with symptomatic severe AS and contraindications or high risk for surgery. To optimize the results of this therapy, accurate patient selection, planning of the procedure and appropriate surveillance at follow-up are essential. Multimodality imaging plays a central role in these steps. The possibilities are numerous and the strengths and limitations of each imaging technique and local expertise and availability are important to select the imaging technique to answer the questions arising at each procedural step (Table). The learning curve and cumulative evidence show the superior accuracy of 3D imaging techniques to size the aortic annulus and select the prosthesis, while refinement in prosthesis design has led to important changes, reducing the invasiveness of the procedure, which is more frequently performed under conscious sedation, fully fluoroscopy guided, and using TTE to evaluate prosthesis function. However, the use of MDCT and CMR at follow-up has provided interesting findings that may have an impact on patient management. Additional studies providing data on the durability of TAVR prostheses will shed light on the incidence of valve thrombosis and infective endocarditis.
Multimodality Imaging Techniques in Transcatheter Aortic Valve Replacement
| Imaging technique | Preprocedural | Periprocedural | Follow-up |
|---|---|---|---|
| Echocardiography (TTE/TEE) | AS severity AV anatomy and degree of calcification Aortic annulus size and root anatomy (3D) Concomitant valvular disease LV function | Guiding catheters Position and deployment of the prosthesis Valve hemodynamics Other procedure related complications (pericardial effusion, myocardial ischemia, aortic dissection, etc.) | Prosthesis deployment and hemodynamics LV function Concomitant valvular disease Valve thrombosis, infective endocarditis (TEE) |
| MDCT | Aortic annulus size and root anatomy AV anatomy and degree of calcification Thoracic aorta, including calcification burden Peripheral arteries LV function C-arm projections | — | Deployment of prosthesis Valve thrombosis (subclinical) Infective endocarditis |
| Cardiac magnetic resonance | Aortic annulus size and root anatomy AV anatomy LV function Thoracic aorta Peripheral arteries | — | Prosthesis deployment and hemodynamics (regurgitation volume) LV function |
| Fluoroscopy | Aortic annulus dimension Peripheral arteries | Guiding catheters Position and deployment of the prosthesis Valve hemodynamics Other procedure-related complications (aortic annulus rupture, coronary ostia occlusion, aortic dissection, etc.) | — |
| Nuclear imaging | — | — | SPECT/CT and 18F-FDG PET/CT in assessment of infective endocarditis |
3D, 3-dimensional; 18F-FDG PET, 18F-fluorodeoxyglucose positron emission tomography; AS, aortic stenosis; AV, aortic valve; CT, computed tomography; LV, left ventricle; MDCT, multidetector row computed tomography; SPECT, single-photon emission computed tomography; TEE, transesophageal echocardiography: TTE, transthoracic echocardiography.
The Department of Cardiology, Heart Lung Center, Leiden University Medical Center (Leiden, The Netherlands) received research grants from Biotronik, Medtronic, Boston Scientific and Edwards Lifesciences. The authors have nothing to disclose.