Individuals with mild to moderately decreased estimated glomerular filtration rate (eGFR=30-59 mL/min/1.73 m2) are considered at high risk of cardiovascular disease (CVD). No studies have compared this risk in eGFR=30-59, diabetes mellitus (DM), and coronary heart disease (CHD) in regions with a low incidence of CHD.
MethodsWe performed a retrospective cohort study of 122 443 individuals aged 60-84 years from a region with a low CHD incidence with creatinine measured between January 1, 2010 and December 31, 2011. We identified hospital admissions due to CHD (myocardial infarction, angina) or CVD (CHD, stroke, or transient ischemic attack) from electronic medical records up to December 31, 2013. We estimated incidence rates and Cox regression adjusted subdistribution hazard ratio (sHR) including competing risks in patients with eGFR=30-59, DM and CHD, or combinations, compared with individuals without these diseases.
ResultsThe median follow-up was 38.3 [IQR, 33.8-42.7] months. Adjusted sHR for CHD in individuals with eGFR=30-59, DM, eGFR=30-59 plus DM, previous CHD, CHD plus DM, and CHD plus eGFR=30-59 plus DM, were 1.34 (95%CI, 1.04-1.74), 1.61 (95%CI, 1.36-1.90), 1.96 (95%CI, 1.42-2.70), 4.33 (95%CI, 3.58-5.25), 7.05 (5.80-8.58) and 7.72 (5.72-10.41), respectively. The corresponding sHR for CVD were 1.25 (95%CI, 1.06-1.46), 1.56 (95%CI, 1.41-1.74), 1.83 (95%CI, 1.50-2.23), 2.86 (95%CI, 2.48-3.29), 4.54 (95%CI, 3.93-5.24), and 5.33 (95%CI, 4.31-6.60).
ConclusionsIn 60- to 84-year-olds with eGFR=30-59, similarly to DM, the likelihood of being admitted to hospital for CHD and CVD was about half that of individuals with established CHD. Thus, eGFR=30-59 does not appear to be a coronary-risk equivalent. Individuals with CHD and DM, or eGFR=30-59 plus DM, should be prioritized for more intensive risk management.
Keywords
The global number of deaths from cardiovascular disease (CVD) increased by 14.5% between 2006 and 2016, and by 53.7% among people over 70 years.1 Coronary heart disease (CHD) and stroke caused a third of all deaths worldwide in 2016.
Decreased renal function (estimated glomerular filtration rate [eGFR]<60 mL/min/1.73 m2) has been associated with an increased risk of all-cause and cardiovascular mortality, with risk rising progressively with decreasing eGFR.2 In fact, individuals with eGFR<60 are more likely to die from cardiovascular causes than from kidney failure.2,3 At the population level, deaths due to reduced eGFR accounted for 3.9% of the total global number of deaths in 2013, and more than half were estimated to occur secondary to CVD.4
In individuals with moderately decreased eGFR (eGFR=30-59), the risk of total and cardiovascular mortality is up to 3 times greater than in individuals with normal eGFR.2 Current international guidelines consider that all individuals with eGFR=30-59 have a high5 or very high6–8 risk of CVD, and recommend aggressive management, in some cases similar to that for established CHD. This increased risk has also been attributed to people with diabetes mellitus (DM).5,8
Nevertheless, only a few studies have analyzed the risk of CHD or CVD or mortality in people with decreased eGFR compared with people with prior CVD or DM, and have shown conflicting results.9-12 Moreover, the results have given rise to concerns that the combination of decreased eGFR and DM could have the highest risk.
In north-east Spain, an area with a low incidence of CHD,5 individuals with type 2 diabetes were reported to have lower cardiovascular risk than coronary patients.13 However, no studies have compared this risk in individuals with decreased eGFR, DM, or CHD. The objective of this study was to compare the added risk of CHD, CVD and total mortality in a population-based cohort of 60- to 84-year-olds with eGFR=30-59, DM, or previous CHD in a region with a low incidence of CHD.5
MethodsData Sources and CohortThe methods used in this project have been reported previously.14 Briefly, this is a retrospective cohort study of 130 233 individuals born in 1950 or earlier who were registered in 40 primary health care centers in the Costa de Ponent Primary Care Service located in the Barcelona metropolitan area (north-east Spain, southern Europe) serving a population of 873 549 individuals, and whose creatinine was measured in a centralized laboratory between January 1, 2010 and December 31, 2011. We excluded individuals with kidney disease stage 5 (eGFR <15, kidney transplant, or dialysis), those receiving health care at home, and those with less than 30 days of follow-up. We collected data on age, sex, smoking status (never smoker, active smoker, and former smoker), hypercholesterolemia (serum cholesterol> 5.2 mmol/L [200 mg/dL] or statin treatment), hypertension [(ICD-10 [tenth revision of the International Classification of Diseases] codes: I10, I15, and subcategories), DM (E11, E12, E14, and subcategories) and previous CVD diagnosis, which included CHD (I20, I21, I22, I23, I24, I25), cerebrovascular disease (G45, G46, I63, I64, I67.8, I67.9, I69), peripheral artery disease (I70, I73, I74), atrial fibrillation (I48), and heart failure (I11.0, I13.0, I13.2, I50); we also collected data on use of statins and renin-angiotensin system drugs.
Serum creatinine levels were measured by a single laboratory using the standardized Jaffe compensated kinetic method traceable to isotope dilution mass spectrophotometry reference method. Estimated glomerular filtration rate was estimated using the creatinine equation from the Chronic Kidney Disease Epidemiology Collaboration15 without correction for race, which was not available.
We excluded individuals with eGFR ≥ 120 because of the disminished precision of estimates at very high eGFR.15 For the purpose of this analysis, to compare the added risk of eGFR=30-59, we excluded individuals with eGFR <30. We focused on individuals younger than <85 years because of the increased risk associated with advanced age, and the sparse evidence on strategies for reducing risk in very old individuals.5
Individuals were stratified in mutually exclusive categories according to the presence of eGFR=30-59, DM and established CHD, or any combinations of the above.
OutcomesIndividuals were followed up from 1 month after the index date until they died, moved to other health system, or until the end of the study (December 31, 2013). Date of death was obtained from hospital or primary care administrative registers without cause specification; all endpoints for CVD were obtained from hospitalization records. The primary endpoint was hospital admittance due to a CHD event (acute myocardial infarction [ICD-9 codes: 410, 412], unstable angina [411] or angina [413]). Any CVD, which included CHD, stroke [ICD-9 codes: 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91], or transient ischemic accident [435] and all-cause mortality were also analyzed as secondary endpoints. The study protocol was approved by the local Clinical Research Ethics Committee (IDIAP Jordi Gol P11/43).
Statistical AnalysisContinuous variables are expressed as mean and standard deviation (for normally distributed variables) or as median and interquartile range (nonnormally distributed variable), and categorical variables as absolute and relative frequencies. To examine differences between groups, we used ANOVA and the Kruskall-Wallis test for normally and nonnormally distributed continuous variables, respectively. We used the chi-square test for categorical variables.
We compared crude rates of CHD, CVD and all-cause mortality incidence per 1000 person-years in patients with eGFR=30-59, DM, and established CHD without decreased eGFR and DM, and combinations of these versus individuals without these diseases.
We used Cox Proportional Hazards models to assess associations between the diseases of interest and CHD events, any CVD and all-cause mortality. For CHD and CVD, we used the competing risks survival techniques (Fine-Gray proportional subdistributional hazard regression) to account for the effect of incident mortality on the estimates. We graphically assessed the assumption of proportionality of the hazard over time (Schoenfeld residuals) for the exposure variable (eGFR; results not shown). For each outcome, models were adjusted for the following variables using a backward stepwise procedure: age, sex, smoking status, hypercholesterolemia, hypertension, atrial fibrillation, heart failure, and the use of statins and renin-angiotensin system drugs. We used incidence curves to compare the risks of CHD, CVD between groups of interest (CIF method), and all-cause mortality (Kaplan-Meier).
We conducted a subanalysis to assess the risk of all events in individuals with mildly decreased eGFR (eGFR=45-59), and a sensitivity analysis of the risk of all events, considering all individuals with established atherosclerotic CVD (stroke, transient ischemic accident, peripheral artery disease) in addition to CHD, as having equivalent CHD risk.
All statistical analyses were performed using R version 3.2.3 (R: a language and environament for statistical computing R Foundation for Statistical Computing, Vienna, Austria), with 2 sided tests and P <.05.
We performed a supplementary subanalysis in individuals aged >85 years.
ResultsOf the initial cohort (130 233 individuals),14 we excluded 14 individuals with eGFR ≥ 120 resulting in 130 219 individuals (122 443 aged <85 years and 7776 aged ≥ 85 years; ).
The median age of 60- to 84-year-olds was 69 [IQR 64-75] years. This age group had a lower percentage of women than the older group (55.3% vs 68.8%, respectively), and a lower prevalence of eGFR <60 (11.4% vs 47.1%, respectively) and cardiovascular risk factors, except DM, and CVD, and drug treatment. Individuals were stratified to baseline groups according to eGFR (30-59), DM or CHD, and combinations (Table 1). Individuals with none of these diseases were younger and had fewer comorbidities and drug treatments than patients with any of these diseases. Those with eGFR=30-59 were older than DM or CHD patients and had a higher percentage of women.
Characteristics of the Study Population According to Disease Group at Baseline (n=113 571; 60- to 84-year-olds)
| Nonen=73 687 | eGFR=30-59n=6981 | DMn=19 960 | eGFR=30-59/DMn=2856 | CHDn=5061 | CHD/ eGFR=30-59n=1100 | CHD/DMn=3105 | CHD/eGFR=30-59/DMn=821 | Poverall | |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | 67.0 [63.0-73.0] | 76.0 [71.0-80.0] | 69.0 [64.0-75.0] | 76.0 [71.0-80.0] | 71.0 [65.0-76.0] | 77.0 [72.0-81.0] | 71.0 [66.0-76.0] | 76.0 [72.0-80.0] | <.001 |
| Sex, female | 44 342 (60.2) | 4388 (62.9) | 10 232 (51.3) | 1768 (61.9) | 1536 (30.3) | 388 (35.3) | 928 (29.9) | 317 (38.6) | <.001 |
| eGFR, mL/min/1.73m2 | 85.9 [76.4-92.4] | 53.1 [46.6-56.9] | 86.1 [76.2-92.6] | 51.1 [44.4-56.2] | 82.0 [72.2-89.2] | 51.9 [45.0-56.5] | 83.2 [72.7-90.5] | 49.0 [42.3-55.0] | <.001 |
| eGFR categories, mL/min/1.73m2 | |||||||||
| > 60 | 73 687 (100) | – | 19 960 (100) | – | 5061 (100) | – | 3105 (100) | – | |
| 45-59 | – | 5535 (79.3) | – | 2089 (73.1) | – | 825 (75.0) | – | 537 (65.4) | |
| 30-44 | – | 1446 (20.7) | – | 767 (26.9) | – | 275 (25.0) | – | 284 (34.6) | |
| Smoking habit | <.001 | ||||||||
| Never smoker | 50 201 (68.1) | 5017 (71.9) | 11 915 (59.7) | 1966 (68.8) | 2402 (47.5) | 579 (52.6) | 1265 (40.7) | 398 (48.5) | |
| Active smoker | 7342 (9.96) | 445 (6.37) | 2159 (10.8) | 178 (6.23) | 497 (9.82) | 82 (7.45) | 319 (10.3) | 53 (6.46) | |
| Former smoker | 16 144 (21.9) | 1519 (21.8) | 5886 (29.5) | 712 (24.9) | 2162 (42.7) | 439 (39.9) | 1521 (49.0) | 370 (45.1) | |
| Hypercholesterolemia | 57 150 (78.8) | 5205 (76.1) | 15 016 (75.8) | 2148 (76.0) | 4593 (91.2) | 961 (87.5) | 2861 (92.4) | 753 (92.1) | <.001 |
| Diabetes mellitus | – | – | 19 960 (100) | 2856 (100) | – | – | 3105 (100) | 821 (100) | <.001 |
| Hypertension | 38 747 (52.6) | 5580 (79.9) | 14036 (70.3) | 2531 (88.6) | 3174 (62.7) | 862 (78.4) | 2236 (72.0) | 695 (84.7) | <.001 |
| Atrial fibrillation | 2503 (3.40) | 628 (9.00) | 908 (4.55) | 312 (10.9) | 466 (9.21) | 190 (17.3) | 312 (10.0) | 129 (15.7) | <.001 |
| CHD | – | – | – | – | 5061 (100) | 1100 (100) | 3105 (100) | 821 (100) | <.001 |
| Stroke | – | – | – | – | 465 (9.19) | 150 (13.6) | 365 (11.8) | 134 (16.3) | <.001 |
| PAD | – | – | – | – | 262 (5.18) | 71 (6.45) | 260 (8.37) | 130 (15.8) | <.001 |
| Heart failure | 1033 (1.40) | 390 (5.59) | 487 (2.44) | 251 (8.79) | 381 (7.53) | 167 (15.2) | 339 (10.9) | 179 (21.8) | <.001 |
| Statins | 25 243 (34.3) | 2791 (40.0) | 11 440 (57.3) | 1711 (59.9) | 4264 (84.3) | 887 (80.6) | 2776 (89.4) | 732 (89.2) | <.001 |
| RASD | 28 788 (39.1) | 4701 (67.3) | 12 556 (62.9) | 2350 (82.3) | 3056 (60.4) | 795 (72.3) | 2335 (75.2) | 704 (85.7) | <.001 |
CHD, coronary heart disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; PAD, peripheral artery disease; RASD, renin-angiotensin system drugs.
Data are expressed as No. (%), mean± standard deviation or median [interquartile range].
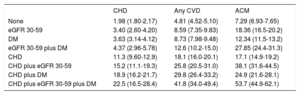
During a median follow-up of 38.3 [IQR, 33.8-42.7] months, 1276 individuals developed a CHD event, 1533 a CVD event, and 3976 individuals died. Crude incidence rates of CHD and CVD events, and death per 1000 person-years are shown in Table 2. The incidence of CHD and CVD was similarly higher in eGFR=30-59 and DM compared with individuals without the diseases, and lower than in individuals with previous CHD, even for the group with both eGFR=30-59 and DM. In contrast, individuals with eGFR=30-59 showed similar crude mortality rates to those with established CHD, and even higher rates when combined with DM. Among patients with established CHD, the incidence of all events increased in the presence of decreased eGFR or DM, with the highest rates observed in individuals with CHD plus eGFR=30-59 and DM.
Crude Incidence Rates Per 1000 Person-years (95% Confidence Interval) of Coronary Heart Disease, Cardiovascular Disease and All-cause Mortality According to Baseline Group (n=113 571; 60- to 84-year-olds) Considering Death as a Competing Risk for CHD and any CVD
| CHD | Any CVD | ACM | |
|---|---|---|---|
| None | 1.98 (1.80-2.17) | 4.81 (4.52-5.10) | 7.29 (6.93-7.65) |
| eGFR 30-59 | 3.40 (2.60-4.20) | 8.59 (7.35-9.83) | 18.36 (16.5-20.2) |
| DM | 3.63 (3.14-4.12) | 8.73 (7.98-9.48) | 12.34 (11.5-13.2) |
| eGFR 30-59 plus DM | 4.37 (2.96-5.78) | 12.6 (10.2-15.0) | 27.85 (24.4-31.3) |
| CHD | 11.3 (9.60-12.9) | 18.1 (16.0-20.1) | 17.1 (14.9-19.2) |
| CHD plus eGFR 30-59 | 15.2 (11.1-19.3) | 25.8 (20.5-31.0) | 38.1 (31.6-44.5) |
| CHD plus DM | 18.9 (16.2-21.7) | 29.8 (26.4-33.2) | 24.9 (21.6-28.1) |
| CHD plus eGFR 30-59 plus DM | 22.5 (16.5-28.4) | 41.8 (34.0-49.4) | 53.7 (44.9-62.1) |
ACM, all-cause mortality; CVD, cardiovascular disease; CHD, coronary heart disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate (mL/min/1.73m2).
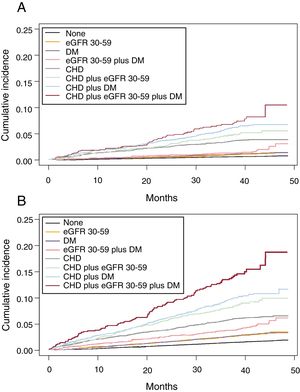
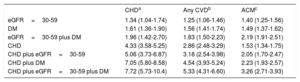
We analyzed the cumulative incidence of admission per 1000 person-years (Figure 1 and ) and multivariate adjusted hazard ratios for hospital admission due to CHD events, CVD and all-cause mortality in individuals with eGFR=30-59, DM or CHD, and combinations, compared with individuals without these diseases (Table 3). eGFR=30-59 and DM carried a similarly increased risk of CHD and CVD and both, individually or combined, were lower than established CHD. However, risk of all-cause mortality was similar for eGFR=30-59, DM and CHD, and significantly higher for the combination of eGFR=30-59 plus DM. In individuals with established CHD, the risk of all events increased significantly in the presence of DM, and even more in individuals with eGFR=30-59 plus DM.
Cumulative incidence function of admission per 1000 person-years due to CHD (A) and CVD (B) in individuals aged 60-84 years with moderately decreased eGFR (30-59 mL/min/1.73 m2), CHD, and DM, and combinations, compared with individuals without these diseases, considering death as a competing risk for CHD and any CVD. CHD, coronary heart disease; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate.
Multivariate Adjusted Subdistribution Hazard Ratio of Admission (95% Confidence Interval) Due to Coronary Heart Disease, Cardiovascular Disease and Hazard Ratio of All-cause Mortality in Individuals Aged 60-84 Years With Moderately Decreased Glomerular Filtration Rate (eGFR=30-59mL/min/1.73 m2), Diabetes Mellitus or CHD and Combinations, compared with Individuals Without These Diseases, Considering Death as a Competing Risk for CHD and any CVD
| CHDa | Any CVDb | ACMc | |
|---|---|---|---|
| eGFR=30-59 | 1.34 (1.04-1.74) | 1.25 (1.06-1.46) | 1.40 (1.25-1.56) |
| DM | 1.61 (1.36-1.90) | 1.56 (1.41-1.74) | 1.49 (1.37-1.62) |
| eGFR=30-59 plus DM | 1.96 (1.42-2.70) | 1.83 (1.50-2.23) | 2.19 (1.91-2.51) |
| CHD | 4.33 (3.58-5.25) | 2.86 (2.48-3.29) | 1.53 (1.34-1.75) |
| CHD plus eGFR=30-59 | 5.06 (3.73-6.87) | 3.18 (2.54-3.98) | 2.05 (1.70-2.47) |
| CHD plus DM | 7.05 (5.80-8.58) | 4.54 (3.93-5.24) | 2.23 (1.93-2.57) |
| CHD plus eGFR=30-59 plus DM | 7.72 (5.73-10.4) | 5.33 (4.31-6.60) | 3.26 (2.71-3.93) |
ACM, all-cause mortality; CVD, cardiovascular disease; CHD, coronary heart disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate.
We performed a subanalysis to assess the risk of all events in the more common category of mildly decreased eGFR (eGFR=45-59). In these individuals, the adjusted multivariate subdistribution HR was 1.28 (95%CI, 0.96-1.70) for CHD events, 1.22 (1.03-1.46) for any CVD, and the HR for mortality was 1.27 (1.12-1.45); for individuals with eGFR=45-59 plus DM, the corresponding HRs were 1.29 (0.83-2.01), 1.56 (1.22-1.99) and 1.97 (1.67-2.32), respectively.
The sensitivity analysis including all individuals with CVD (), and individuals aged ≥ 85 years yielded similar results ().
DiscussionIn this population-based cohort of 60- to 84-year-olds in a region with a low incidence of CHD, crude rates and the adjusted risk of hospital admission for CHD and CVD events in individuals with eGFR=30-59 were similarly high to those in individuals with DM. Both, alone or combined, were lower than those in individuals with previous CHD. The incidence and adjusted risk were closer to that of established CHD for CVD when eGFR=30-59 plus DM was considered. There were no differences in all-cause mortality between individuals with eGFR=30-59, DM or established CHD. Moreover, in patients with established CHD, DM substantially increased the risk of CHD events, which was highest in individuals with CHD plus DM and eGFR=30-59.
To our knowledge, ours is the first study to compare cardiovascular risk between individuals with eGFR=30-59, DM and CHD in a region with a low incidence of CHD. Our results confirm the higher cardiovascular risk associated with eGFR=30-59 compared with a normal eGFR level, similar to individuals with DM. However, this increased risk is lower than that in patients with established CHD. Previously, another study in the same area found that individuals with DM had a lower CHD incidence and 10-year cardiovascular mortality than patients with a first acute myocardial infarction (MI) without diabetes.13
Our results are comparable to those of similar study in Canada, which also reported higher adjusted relative rates of myocardial infarction in individuals with decreased eGFR (1.4; 95%CI, 1.3-1.5), DM (2.0; 95%CI, 1.9-2.1), and decreased eGFR plus DM (2.7; 95%CI, 2.5-2.9), and lower than in individuals with established CHD (3.8; 95%CI, 3.5-4.1).10 However, these authors also reported no differences in all-cause mortality.
Some studies have compared rates of cardiovascular death in these groups. One study in individuals aged 45-64 years old observed a 60% lower risk of cardiovascular death in individuals with eGFR=30-59 (0.39; 95%CI, 0.24-0.62) compared with those with prior MI.12 More recently, the risk of cardiovascular death was found to be increased more than 2-fold in individuals aged 40-65 years old with eGFR <60 (2.31; 95%CI, 1.91-2.71), more than 4-fold in participants with diabetes mellitus (4.62; 95%CI, 4.13-5.11), and more than 6-fold in participants with CVD (6.78; 95%CI, 6.16-7.4) compared with individuals without these diseases.11 There were no differences in risk in individuals with eGFR <60 and DM without CVD (10.03; 95%CI, 6.47–13.6) and those with CVD,11 which we did not find in eGFR=30-59. However, in individuals aged> 65 years, no differences in risk of cardiovascular death were observed between individuals with eGFR <60 and those with previous MI, even though the former group had a lower risk of CVD, suggesting that case-fatality rates may be higher in individuals with decreased eGFR.9 Inequalities in cardiovascular treatment could also explain the worse prognosis, and mask differences in mortality risk. The broad implementation of interventional cardiology and preventive secondary measures has improved prognosis in patients with established CHD.16 However, while the post-MI prognosis in patients with renal dysfunction has also significantly improved in the last decade,17 there have been reports of a lower frequency of referral for coronary angiography and treatment with guideline-based cardiovascular medications.18–20 Interestingly, some authors have reported that, after adjusting for eGFR, they observed a reduced difference between sexes in in-hospital and long-term mortality following ST-segment elevation MI.21
In patients with established CHD, we observed a further increase in crude incidence rates and risk of all outcomes with DM, especially with DM plus eGFR=30-59, which has been reported previously in eGFR <60 for cardiovascular death.11 Most cardiovascular events occur in patients with established CVD,22,23 but not all patients have the same risk of new events.22–24 Moreover, in clinical practice, many CHD patients do not achieve the guideline standards for secondary prevention.25,26 It is therefore important to identify the highest-risk patients, who are most likely to benefit from a more intensive approach. DM is a known risk factor for short and long-term prognosis after CV events.5,23,24,27 Similarly, renal insufficiency has also been found to be a a predictor of MI, stroke or cardiovascular death, and all-cause-mortality at 1 and 2 years and in the long-term following acute coronary syndrome.21,23,28 The presence of diabetes and chronic kidney disease have been found to increase the risk of all-cause mortality and nonfatal myocardional or coronary death at 5 years in different subtypes of stable CHD.24 Our study confirms that, in a southern European population, CHD patients with DM or decreased eGFR and DM are at higher risk of CHD and CVD events than CHD patients without these diseases, and may therefore benefit from more intensive treatment.
LimitationsOur results must be considered in light of some study limitations. First, regarding measurements of renal function, individuals were classified according to only 1 eGFR measurement, which is common in epidemiological studies,2,3,9–11 and eGFR was estimated from serum creatinine measurements. Creatinine-based estimating formulas may have some limitations, and specifically Chronic Kidney Disease Epidemiology Collaboration,15 even though this is the recommended6 and most widely used formula in clinical settings. We were also unable to evaluate the effect of proteinuria on cardiovascular risk, as data were only available for 28% of the cohort; nevertheless, eGFR and proteinuria have been shown to have independent prognostic implications.2 The main analysis was limited to individuals aged 60-84 years, which includes most individuals with moderately decreased eGFR. A subanalysis in individuals aged ≥ 85 years yielded similar results. Although it is not certain that our results can be extrapolated to young individuals, in which decreased eGFR has probably different etiologies, we believe it can be applied to individuals aged ≥ 50 years.
Other limitations are related to the use of electronic health records. Data for CVD in primary health care has been shown to be of higher quality than for other diseases, and suitable for epidemiological studies in our population.28,29 We were unable to evaluate fatal CVD outside the hospital and cardiovascular mortality. While we focused our primary analysis on CHD events, including myocardial infarction or angina, the results were similar when we included cerebrovascular outcomes, and in patients with established CHD or other atherosclerotic CVD. All models were adjusted for baseline cardiovascular risk factors and diseases, as well as for use of statins and renin-angiotensin-system treatment, but not for other drugs such as aspirin, time since diagnosis with DM, cardiovascular risk factor control, invasive cardiologic treatments, and other comorbidities or socioeconomic factors that may affect the incidence or prognosis of cardiovascular events, either initially or during follow-up. Moreover, some factors such as neoplasms, infections and other clinical events can modify eGFR and affect all-cause mortality, although these data were not available; to reduce this effect, we excluded individuals with <30 days of follow-up. Although some degree of residual counfounding may exist, we believe that this effect would be minimized by our large sample size.
Despite these limitations, we believe that our results are an appropiate proxy for the total cardiovascular disease burden and add new information about cardiovascular risk among individuals with decreased eGFR, DM and CHD in a southern region with low cardiovascular risk. The main strength of the study is the very large community-based sample, reflecting the population in real-world practice. Moreover, creatinine determinations were performed using the recommended calibrated Isotope dilution mass spectrometry method,6 and in a centralized laboratory, which reduces variability.
CONCLUSIONSIn our study of a population-based cohort of 60- to 84-year-olds in a region with a low incidence of CHD, the adjusted higher risk of hospital admission for CHD and CVD in individuals with eGFR=30-59 was comparable to that in individuals with DM, and ∼50% lower than that in those with established CHD.
Our results have important implications for clinical practice in the region. According to these results, individuals aged 60-79 years with eGFR=30-59, and especially the more common and mildest eGFR decrease (= 45-59), should probably not be considered as coronary-risk equivalent based solely on eGFR. Moreover, individuals with established CHD and either DM or eGFR=30-59, or both, had a further increased risk of new events and should be prioritized for more intensive risk management.
Further research is needed to better understand how to manage risk in individuals with eGFR=30-59, especially in combination with DM, and to evaluate the prognosis and use of interventional and other acute cardiovascular treatments in individuals with decreased eGFR and CHD.
- –
Current international guidelines consider all individuals with mild to moderately decreased eGFR (30-59 mL/min) at high or very high risk of CVD, and recommend aggressive management, in some cases similar to that for established CHD.
- –
This increased risk has also been attributed to people with DM.
- –
Few studies have compared the risk of CHD in people with eGFR 30-59, DM, or prior CHD.
- –
In a previous study in north-east Spain, an area with a low incidence of CHD, individuals with type 2 diabetes were reported to have lower cardiovascular risk than CHD patients.
- –
In a region with a low incidence of CHD, 60- to 79-year-olds with moderately impaired renal function (eGFR=30-59) had a similar risk of CHD and CVD events to that in individuals with DM.
- –
The risk was 50% lower than in individuals with established CHD.
- –
In individuals with CHD, the presence of DM or eGFR=30-59 plus DM significantly increased the risk of new events.
This project was supported by a research grant from the Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), awarded under the 2011 call of the Health Strategy Action, within the National Research Program oriented to Societal Challenges. This program forms part of the Technical, Scientific and Innovation Research National Plan 2008-2011, co-funded by European Union ERDF funds (European Regional Development Fund) (PI11/02220).
Ministry of Economy and Competitiveness through the Carlos III Institute of Health (Red RedIAPP RD12/0007) and European Regional Development Funds (ERDF-FEDER) Generalitat de Catalunya through the Agency for Management of Universities and Research Grants (AGAUR) [2014 SGR 1225] [2014 SGR 902].
CONFLICTS OF INTERESTNone declared.
MACAP Renal Costa de Ponent Research Group: Eva Alonso-Bes, Virtudes Álvarez-Funes, Sílvia Cobo-Guerrero, Esther Freixes-Villaró, Roser Güell-Miró, Luisa Pascual-Benito, Maria Soler-Vila