Lipid-lowering therapy is one of the cornerstones of cardiovascular prevention and is one of the most effective strategies in the secondary prevention of ischemic heart disease. Nevertheless, the current treatment of lipid disorders, together with lifestyle changes, fails to achieve the targets recommended in clinical guidelines in a substantial proportion of patients. PCSK9 inhibitors have demonstrated safety and efficacy in the treatment of dyslipidemia. Due to their ability to reduce low-density lipoprotein cholesterol levels, these drugs have recently been approved for clinical use by Spanish regulatory agencies, with the aim of reducing cardiovascular risk in selected patient groups.
Keywords
Although safe, effective drugs are available for reducing circulating cholesterol concentrations, several epidemiologic studies have reported that most patients at high cardiovascular risk have low-density lipoprotein cholesterol (LDLc) values above the target levels recommended in clinical guidelines.1 This is an important issue, considering that patients reaching the therapeutic target have a lower risk of cardiovascular complications and death.2 There are several reasons for poor cholesterol control, such as poor treatment adherence among patients and treatment delays among physicians, but sometimes poor control is the result of ineffective lipid-lowering therapy.
In this regard, various patient subgroups may have difficulty reaching LDLc goals with the currently available drugs.3 These include the population of patients with familial hypercholesterolemia (FH), who generally show extraordinarily high LDLc concentrations that are difficult to reduce to recommended levels despite optimal lipid-lowering therapy,4,5 patients with partial or total statin intolerance, those unable to receive the recommended drugs,6 and numerous patients at very high cardiovascular risk who may require marked LDLc reductions that cannot be attained because of the considerable variability in the response to statins.7 Hence, it seems evident that new potent lipid-lowering agents are needed to replace statin therapy or be combined with these drugs.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a protein that regulates cholesterol metabolism by controlling degradation of low-density lipoprotein (LDL) receptors.8 After binding to an LDL particle, the LDL receptor internalizes into the cytoplasm within structures known as endosomes. The LDL-LDL receptor complex then dissociates: LDL is processed for degradation within the lysosomes, whereas the LDL receptors are recycled toward the cell surface where they bind to new LDL particles. This recycling process occurs approximately 150 times for each receptor. LDL receptors that are bound to a PCSK9 molecule cannot be recycled and are instead processed for lysosomal degradation together with LDL particles. Thus, PCSK9 inhibition reduces the number of receptors that will be degraded and increases their numbers on the cell surface, which lowers the plasma cholesterol concentration.
Two human monoclonal antibodies able to block PCSK9, alirocumab and evolocumab, are currently available in Spain. These agents, which are administered by subcutaneous injection every 2 or 4 weeks, are able to immediately (within hours) block the circulating PCSK9. Reductions in LDLc are perceptible at 48hours after the start of treatment. Anti-PCSK9 antibodies reduce cholesterol concentrations in a dose-dependent manner, achieving decreases of up to 60% at a dose of 140 mg of evolocumab or 150 mg of alirocumab, administered every 2 weeks, with no significant adverse effects to date.9 In addition to reducing LDLc, both drugs can lower lipoprotein (a) levels by 20% to 30% and moderately lower triglyceride concentrations, with marginal elevations of high-density lipoprotein cholesterol levels. These reductions are cumulative to those obtained with statins or any other lipid-lowering drug.
Evolocumab and alirocumab have been approved by the Spanish Ministry of Health to be dispensed in the hospital setting for patients with the following indications, based on therapeutic positioning reports: patients with FH and LDLc plasma concentrations >100 mg/dL despite the use of maximum statin doses, patients with established cardiovascular disease (ischemic heart disease, ischemic cerebrovascular disease, and peripheral arterial disease) and uncontrolled LDLc (>100 mg/dL) with maximum tolerated statin doses, and any patients within these 2 groups who are intolerant to or have contraindications for statin use, and whose LDLc levels are >100mg/dL.
The Spanish Society of Cardiology has decided to establish a series of recommendations to position this therapeutic option, while being aware that clinical trials investigating the related morbidity and mortality are needed to provide a definitive basis for the indications. The complete document derived from this study is available on the Spanish Society of Cardiology website.10
The patient populations with priority to receive PCSK9 treatment and the LDLc concentrations above which PCSK9 would be indicated were established by consensus at participatory meetings. PCSK9 should probably only be used in patients who have already received optimal lipid-lowering therapy; that is, potent statins at the maximum tolerated doses with or without ezetimibe (if a patient exclusively treated with statins is far from achieving the therapeutic goals, anti-PCSK9 can be started if the attending physician considers that these goals will not be reached solely with the addition of ezetimibe).
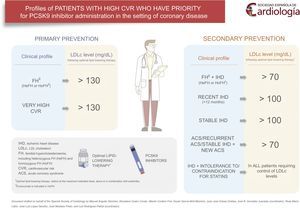
PATIENTS IN SECONDARY PREVENTIONPatients With Familial Hypercholesterolemia and Ischemic Heart Disease, Showing Low-density Lipoprotein Cholesterol >70 mg/dL Despite Optimal Lipid-lowering TherapyFamilial hypertension (diagnosed clinically or genetically) is associated with a high risk of premature coronary disease and death.11 Patients with FH and cardiovascular disease have a very high risk of new complications.12 Although there are no data in this specific population, strict control of LDLc values to below 70 mg/dL improves prognosis following an acute coronary syndrome (ACS).12
Patients With Recent Ischemic Heart Disease (<12 Months) and Low-density Lipoprotein Cholesterol >100 mg/dL, Despite Optimal Lipid-lowering TherapyThis profile refers to patients diagnosed with ischemic heart disease during the previous year with persistent LDLc concentrations >100 mg/dL despite optimal lipid-lowering therapy. This profile confers a particularly elevated risk because of the recent diagnosis of coronary disease, as the incidence of new events is higher in the first few months after diagnosis, especially in patients who have experienced an ACS.13 Within this group, patients at highest risk (ie, those with the most recent diagnosis and LDL values farthest from the therapeutic target) are considered to be those who will gain the greatest benefits.
Patients With Stable Ischemic Heart Disease and Low-density Lipoprotein Cholesterol >100 mg/dL Despite Optimal Lipid-lowering TherapyPatients with stable coronary disease, including those with stable chronic angina and stabilized patients who have experienced a documented ACS (myocardial infarction with or without coronary revascularization therapy) more than 1 year previously, also have a high risk of new cardiovascular complications and death, although the risk is lower than that of patients with a recent ACS.14 Clinical practice guidelines on dyslipidemia and cardiovascular prevention consider this profile to confer very high cardiovascular risk.15 As in the previous profile, and although the risk of cardiovascular complications is not as high, initiation of anti-PCSK9 antibody treatment may also be indicated when LDLc values exceed 100 mg/dL.
Patients With a Recurrent Acute Coronary Syndrome and Those With Chronic Ischemic Heart Disease Who Experience an Acute Coronary Syndrome and Who Fail to Reach the LDLc Therapeutic Target of <70 mg/dL Despite Optimal Lipid-lowering TherapyPatients hospitalized for an ACS, defined as an ST-segment elevation acute myocardial infarction, a non–ST-segment elevation acute myocardial infarction, or unstable angina, do not have a benign long-term prognosis. Furthermore, if the ACS is recurrent, there is a greater risk of in-hospital and out-of-hospital cardiovascular complications and death.16 In a retrospective study conducted in Spain and including 4345 patients who survived the hospital phase of an ACS, 21.5% had the combined event of cardiovascular death, acute myocardial infarction, or stroke over a 5-year follow-up. The risk was particularly high in the first year, when 34.8% of all events recorded during the entire observation period occurred.17 Clinical registries in large databases, which indicate the real-world situation, have cited 7-year mortality rates of 31% for men and 47% for women in the case of a first acute myocardial infarction, and 58% for men and 74% for women in recurrent acute myocardial infarction.14,16 These data demonstrate the high risk of recurrent disease and death in ACS patients and indicate the need to implement optimal cardiovascular protection measures, particularly in patients with more than 1 ACS episode and those with stable ischemic heart disease and an ACS episode. Therefore, the use of PCSK9 inhibitors is a priority in these patients if they do not achieve the LDLc therapeutic goal of <70 mg/dL despite maximum tolerated doses of oral lipid-lowering drugs.
Patients With Ischemic Heart Disease Who Have Not Reached the Therapeutic Target Because of Contraindications or Demonstrated Statin IntoleranceA subgroup of patients with ischemic heart disease do not achieve the therapeutic goal because of partial or complete statin intolerance. According to several studies, this group comprises approximately 15% of all patients with an indication for this treatment.6 In addition, it has been proven that discontinuation of statin therapy is associated with worsening of the cardiovascular prognosis in coronary patients.18 Therefore, in ischemic heart disease patients not reaching the LDLc therapeutic goal because of intolerance to high-dose statins and in those with contraindications for these drugs, the indication for PCSK9 inhibitors is probably justified to achieve the LDLc target value.
PATIENTS IN PRIMARY PREVENTIONPatients With Familial Hypercholesterolemia and No Cardiovascular Diseases Who Have Low-density Lipoprotein Cholesterol Values >130 mg/dL Despite Optimal Lipid-lowering TherapyAs was mentioned above, FH is the most common hereditary condition associated with premature coronary disease.11 When FH remains untreated, the risk of experiencing a myocardial infarction before the age of 60 years is approximately 50% in men and 30% in women.12 Transmission of FH is by an autosomal-dominant inheritance mechanism with high penetrance that is expressed since birth. The condition is mainly produced by mutations in the LDL receptor gene and less commonly by apolipoprotein B and PCSK9 mutations. The prevalence of FH in the general population is approximately 1 in every 200 to 500 persons. In Spain, an estimated 100 000 individuals have FH, and most of them have not received a diagnosis. Clinically, FH is characterized by very high LDLc values, a history of hypercholesterolemia in first-degree relatives, premature cardiovascular disease, and the presence of tendon xanthomas and corneal arcus. The diagnosis can be suspected on the basis of clinical criteria and confirmed by genetic study. To promptly identify affected relatives of a diagnosed case, cascade family screening is recommended, using LDLc determination and, when available, genetic study.
According to the latest guidelines of the European Atherosclerosis Society and European Society of Cardiology,15 patients with FH should be considered at high cardiovascular risk. Therefore, the aim should be to reduce LDLc with administration of statins, ezetimibe, or bile acid sequestrants to below 100 mg/dL or by at least 50% of the baseline value. Anti-PCSK9 antibodies may be used when the LDLc concentration is greater than 130 mg/dL.
Patients Without Cardiovascular Disease but at Very High Cardiovascular Risk, With Low-density Lipoprotein Cholesterol Values >130mg/dL Despite Optimal Lipid-lowering TherapyTo be included in this profile, patients must meet the necessary criteria to be considered at high cardiovascular risk according to the European guidelines on dyslipidemia (type 2 or type 1 diabetes mellitus with target organ damage, estimated glomerular filtration rate <60 mL/min/1.73 m2, or a SCORE value >10%).19 This profile probably also includes individuals with severe dyslipidemia other than FH, very high baseline cholesterol values, and a persistent LDLc concentration >130 mg/dL despite optimal lipid-lowering therapy, such as individuals with familial combined hyperlipidemia or severe polygenic forms.
CONCLUSIONSWith the availability of new, highly effective lipid-lowering drugs such as PCSK9 inhibitors, the recommended LDLc target levels will be reached in a large number of patients (Figure). It is anticipated that the use of these agents will significantly decrease overall cardiovascular risk and mortality. Completion of ongoing clinical trials using several of these new monoclonal antibodies and investigation of morbidity and mortality will enable more accurate delineation of patient populations that will benefit from this therapy and will provide cost-effectiveness analyses.
Although the situations described in this document refer exclusively to atheromatous coronary heart disease, the same conclusions can be applied to other atherosclerosis-related conditions, such as peripheral arterial disease and cerebrovascular disease.
CONFLICTS OF INTERESTM. Anguita Sánchez has received honoraria from Amgen for writing/revising manuscripts and for presentations. A. Cordero has received honoraria for consultancy work, expert testimony, and presentations from MSD, AstraZeneca, Menarini, Amgen, and Sanofi. J.M. Mostaza has received honoraria for consultancy work, talks, and educational presentations from Amgen and Sanofi. R.M. Lidón has received honoraria for consultancy work from AstraZeneca, and for expert testimony and presentations from Amgen and Ferrer. J.L. López-Sendón has received grants from Sanofi, Pfizer, Bayer, and GlaxoSmithKline, honoraria for participation in manuscript revisions from Pfizer, Bayer, and Merk, for writing/revising manuscripts from Amgen, for consultancy work from Bayer, and for presentations from Menarini, Servier, Novartis, and Merck, and funding for travel expenses from Servier. X. García-Moll has received honoraria for presentations from Sanofi, Amgen, and MSD, and funding for travel expenses from Sanofi. J.J. Gómez Doblas has received honoraria for presentations from Amgen. A. Castro has received honoraria for consultancy work from GOC Networking and for presentations from Amgen. L. Rodríguez Padial has received honoraria for par ticipation in manuscript revisions from Amgen and Sanofi and for manuscript preparation from Rovi, Amgen, and MSD. J.R. González-Juanatey has received honoraria for presentations from Amgen, Sanofi, MSD, Boehringer-Ingelheim, Novartis, AstraZeneca, Ferrer, Servier, Bayer, and Menarini.