Pulmonary regurgitation (PR) is a frequent complication after repair of congenital heart disease. Lymphocyte expression of adrenoceptors (β1 and β2) and kinases (GRK2, GRK3, and GRK5) reflects the neurohumoral changes that occur in heart failure (HF). The main objective of this study was to describe the gene expression of these molecules in circulating lymphocytes in patients with severe PR.
MethodsA prospective study was conducted to analyze lymphocyte expression of these molecules in patients with severe PR and compare it with expression in healthy controls and patients with advanced HF.
ResultsWe studied 35 patients with severe PR, 22 healthy controls, and 13 patients with HF. Multiple comparisons analysis showed that β2-adrenoceptor gene expression levels were higher in the control group than in patients in the PR and HF groups and that expression in the latter 2 groups was similar (748.49 [rank 1703.87] vs 402.80 [rank 1210.81] vs 287.46 [rank 685.69] P = .001). Similar findings were obtained in gene expression of GRK2 (760.89 [rank 1169.46] vs 445.17 [rank 1190.69] vs 284.09 [rank 585.27] P < .001). There were no differences in expression levels of these molecules according to clinical variables in patients with PR.
ConclusionsThe gene expression pattern of GRK2 and β2-adrenoceptor as molecular markers of cardiac dysfunction was altered in patients with severe PR compared with controls and was similar to expression in patients with advanced HF.
Keywords
Chronic pulmonary regurgitation (PR) is a frequent complication in patients who have undergone surgical repair of congenital heart disease. Over time, the regurgitation causes right ventricular (RV) volume overload and progressive dysfunction,1 which are associated with reduced exercise capacity and increased risk of arrhythmias and sudden cardiac death.2
Although pulmonary valve replacement (PVR) is a low-risk intervention,3 multiple reinterventions are required due to subsequent degeneration of the implanted bioprosthesis or homograft.4 Clinical practice guidelines5,6 recommend surgery for severe PR in symptomatic patients or those with moderate-severe RV dilatation/dysfunction.7,8 However, most of the series analyzed showed no evidence of a postoperative increase in exercise capacity or RV systolic function or a prognostic improvement,9 and consequently many authors recommend earlier performance of the PVR.10
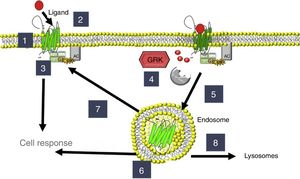
Homeostatic regulation of the cardiovascular system is controlled by the autonomic nervous system, with continuous interaction between the 2 systems. Heart failure (HF) patients have increased concentrations of circulating catecholamines, which alter the expression and activity of adrenoceptors and G protein-coupled receptor kinases (GRKs); agonist binding to adrenoceptors triggers their activation and phosphorylation by GRKs. Several isoforms (GRK2, GRK3, and GRK5) are found in the heart and in circulating mononuclear leukocytes (CMLs); once these kinases phosphorylate β-adrenoceptors, they are internalized into the cell and are no longer active in the cell membrane11,12 (Figure 1). The expression of β-adrenoceptors and GRKs (particularly GRK2 and GRK5) is altered by the hemodynamic changes occurring in HF13–16 and can thus be used for disease monitoring. Initially, these changes in the expression of β-adrenoceptors and GRKs were determined in material of cardiac origin (endomyocardial biopsies, cardiac explants), but their expression can also be studied in CMLs,15,17,18 allowing the use of these cells in peripheral blood samples as biomarkers. Indeed, because the CML expression of GRK219 has been used as a molecular marker in the study of HF, a similar approach could be useful in PR.
Regulation of adrenoceptors and G protein-coupled receptor kinases (GRKs). 1: β-adrenoceptor location; 2: activation; 3: signaling through G proteins with adenylate cyclase (AC) activation; 4: phosphorylation by GRKs; 5: endocytosis; 6: location of endosomes; 7: recycling to the membrane; 8: degradation in lysosomes.
In the present work, a molecular approach was used to improve the understanding of the pathophysiological process underlying PR and to help establish the optimal moment for PVR. Our main objective was to determine the CML expression of adrenoceptor and GRK genes in patients with severe PR as a sequela of congenital heart disease surgery and to compare their expression levels with those of controls and patients with HF. Secondary objectives were to compare the expression of adrenoceptors and GRKs among patients with severe PR according to the presence of symptoms and RV dysfunction or significant dilatation, because these are the surgical indications.
METHODSPatients diagnosed with severe PR followed up in the Adult Congenital Heart Disease Unit were prospectively selected from December 2011 to July 2015. Severe PR was diagnosed according to standard echocardiographic criteria20 and was confirmed by cardiac magnetic resonance imaging. Patients with PR and left ventricular dysfunction or another severe valve disease were excluded.
After participants signed an informed consent form, a peripheral blood sample was taken for analysis of the CML gene expression of adrenoceptors and GRKs. Clinical (New York Heart Association [NYHA] functional class) and electrocardiographic (heart rate and QRS width) data were also collected and RV assessment was performed via cardiac magnetic resonance imaging (ventricular ejection volume and fraction).
The study included 22 healthy volunteers, matched for age and sex with the included patients, and 13 patients with advanced HF of nonischemic origin; these individuals also underwent a single blood extraction for the analysis of adrenoceptor and GRK gene expression. Patients with inflammatory processes that could alter the expression of these molecules were excluded.
The study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Hospital Universitario y Politécnico de La Fe.
Analysis of Adrenoceptor and GRK Gene Expression in Peripheral Blood Circulating Mononuclear LeukocytesThe biological material studied was a sample of fresh blood (10mL) obtained by venipuncture and preserved in a tube with anticoagulant (EDTA). Immediately after the extraction, CMLs were isolated using a Ficoll gradient.21 Samples were stored at –80°C until processing. In a first phase, RNA was isolated by centrifugation according to the Tripure Isolation Reagent protocol (Roche). Once the RNA was isolated, quality assurance was carried out with the StdSens RNA Experion system (Bio-Rad), which assesses sample quality, purity, and integrity, setting as a requirement an RNA quality indicator (RQI) > 0.75. In a second phase, real-time quantitative reverse transcription-polymerase chain reaction was performed with the TaqMan Gene system in a GeneAmp 7500 Fast System thermocycler (Applied Biosystems) following a previous experimental protocol.21 The mRNA encoding the adrenoceptors and GRKs was quantified, with glyceraldehyde 3-phosphate dehydrogenase as internal control, with the following TaqMan probes: β1-AR, Hs00265096_s1; β2-AR, Hs00240532_s1; GRK2, Hs00176395_m1; GRK3, Hs00178266_m1; GRK5, Hs00178389_m1; and glyceraldehyde 3-phosphate dehydrogenase, Hs99999905_m1 (Applied Biosystems).
Statistical AnalysisQualitative variables are expressed as percentage and quantitative variables as mean ± standard deviation. Adrenoceptor and GRK levels are expressed as median (range) because they failed to meet normality assumptions. Three groups were analyzed: controls, patients with severe PR, and patients with advanced HF. A descriptive analysis was performed and the expression levels of adrenoceptors and GRKs in the 3 groups were compared by a Kruskal-Wallis test using multiple comparisons among groups, given the sample size and the distribution of the gene expression variables.
Subsequently, the expression levels of adrenoceptors and GRKs were compared within the group of patients with PR according to their clinical status and the RV assessment data through the Wilcoxon nonparametric W test.
P < .05 was considered statistically significant. SPSS 15 and STATA 13.1 statistical packages were used for all analyses.
RESULTSStudy PopulationThirty-five patients with severe PR were included (54.3% men; mean age, 32.2 years). Regarding the underlying heart disease, 65.7% had tetralogy of Fallot and 25.7% had valvular pulmonary stenosis; additionally, 1 patient had valvular agenesis and 2 patients underwent interventions for other heart diseases that led to PR during follow-up. The pulmonary valve had been repaired in 94.3% of the patients. A myectomy/valvulotomy alone was performed in 8.6%, and a transannular patch or RV outflow tract augmentation was placed in 82.9%.
At baseline, 77.1% of patients were in NYHA functional class I; 91.4% were in sinus rhythm, with a mean QRS duration of 142.4 ± 29.3 milliseconds. Additionally, 37.1% had RV dysfunction according to cardiac magnetic resonance imaging criteria (RV ejection fraction < 45%) and 65.7% had significant RV dilatation (RV end-diastolic volume index on cardiac magnetic resonance imaging ≥ 150mL/m2).
Comparisons were made with 22 controls (62% men; mean age, 36.7 years) and 13 patients with advanced HF (76.9% men; mean age, 47.5 years). Patients with HF had nonischemic cardiomyopathies (mean left ventricular end-diastolic diameter, 67.6 ± 13.7mm; mean left ventricular end-systolic diameter, 57.5 ± 15mm), with a mean left ventricular ejection fraction of 22.5% ± 16.6%.
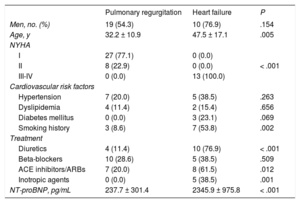
The clinical characteristics of the patients with PR and HF are compared in Table 1. Patients with PR had a better clinical status and lower levels of N-terminal pro-B type natriuretic peptide. The beta-blocker treatment rate in the HF group was low because these patients were at an advanced disease stage, with poor hemodynamic tolerance.
Baseline Characteristics of Patients with Severe Pulmonary Regurgitation and Heart Failure
| Pulmonary regurgitation | Heart failure | P | |
|---|---|---|---|
| Men, no. (%) | 19 (54.3) | 10 (76.9) | .154 |
| Age, y | 32.2 ± 10.9 | 47.5 ± 17.1 | .005 |
| NYHA | |||
| I | 27 (77.1) | 0 (0.0) | |
| II | 8 (22.9) | 0 (0.0) | < .001 |
| III-IV | 0 (0.0) | 13 (100.0) | |
| Cardiovascular risk factors | |||
| Hypertension | 7 (20.0) | 5 (38.5) | .263 |
| Dyslipidemia | 4 (11.4) | 2 (15.4) | .656 |
| Diabetes mellitus | 0 (0.0) | 3 (23.1) | .069 |
| Smoking history | 3 (8.6) | 7 (53.8) | .002 |
| Treatment | |||
| Diuretics | 4 (11.4) | 10 (76.9) | < .001 |
| Beta-blockers | 10 (28.6) | 5 (38.5) | .509 |
| ACE inhibitors/ARBs | 7 (20.0) | 8 (61.5) | .012 |
| Inotropic agents | 0 (0.0) | 5 (38.5) | .001 |
| NT-proBNP, pg/mL | 237.7 ± 301.4 | 2345.9 ± 975.8 | < .001 |
ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; NT-proBNP, N-terminal pro-B type natriuretic peptide; NYHA, New York Heart Association.
Values represent No. (%) or mean ± standard deviation.
*Chi-squared test/Fisher exact test for qualitative variables, and Mann-Whitney U test for qualitative variables.
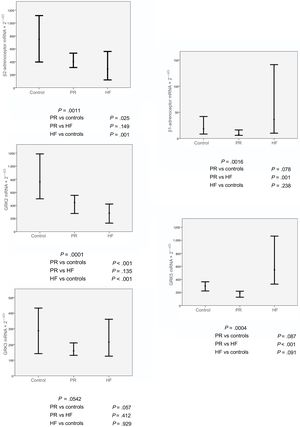
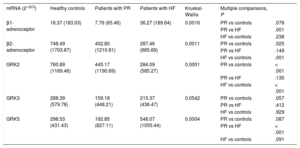
The expression levels of adrenoceptors and GRKs in CMLs obtained from blood samples were compared among the 3 study groups: controls, patients with severe PR, and patients with HF. The median (range) mRNA values of the adrenoceptors and GRKs are shown in Table 2 according to study group.
Adrenoceptor and GRK Expression According to Study Group
| mRNA (2–ΔCt) | Healthy controls | Patients with PR | Patients with HF | Kruskal-Wallis | Multiple comparisons, P | |
|---|---|---|---|---|---|---|
| β1-adrenoceptor | 18.37 (183.03) | 7.76 (65.46) | 36.27 (189.64) | 0.0016 | PR vs controls | .078 |
| PR vs HF | .001 | |||||
| HF vs controls | .238 | |||||
| β2-adrenoceptor | 748.49 (1703.87) | 402.80 (1210.81) | 287.46 (685.69) | 0.0011 | PR vs controls | .025 |
| PR vs HF | .149 | |||||
| HF vs controls | .001 | |||||
| GRK2 | 760.89 (1169.46) | 445.17 (1190.69) | 284.09 (585.27) | 0.0001 | PR vs controls | < .001 |
| PR vs HF | .135 | |||||
| HF vs controls | < .001 | |||||
| GRK3 | 288.39 (579.78) | 159.18 (448.21) | 215.37 (436.47) | 0.0542 | PR vs controls | .057 |
| PR vs HF | .412 | |||||
| HF vs controls | .929 | |||||
| GRK5 | 298.53 (431.43) | 192.85 (827.11) | 548.07 (1055.44) | 0.0004 | PR vs controls | .087 |
| PR vs HF | < .001 | |||||
| HF vs controls | .091 |
GRKs, G protein-coupled receptor kinases; HF, heart failure; mRNA, messenger RNA; PR, pulmonary regurgitation.
As shown in Figure 2, and according to the results of the multiple comparison analysis, significant differences were found in the expression of the β2-adrenoceptor in the control group vs the PR and HF patient groups: 748.49 (rank, 1703.87) vs 402.80 (rank, 1210.81) vs 287.46 (rank, 685.69) (P = .001). The expression of β2-adrenoceptor was significantly lower in the PR and HF groups than in the control group (PR vs control, P = .025; HF vs control, P = .001). Similar results were obtained for the gene expression of GRK2, with significantly higher GRK2 mRNA values in the control group than in the PR and HF groups: 760.89 (rank, 1169.46) vs 445.17 (rank, 1190.69) vs 284.09 (rank, 585.27) (P < .001); this finding was confirmed in the results of the group analysis (PR vs controls, P < .001; HF vs controls, P < .001).
In contrast, no differences were observed among the groups in terms of GRK3 gene expression. Furthermore, although significant differences were found in the expression of the β1-adrenoceptor and GRK5 in the statistical analysis, the multiple comparison analysis found no differences in the expression of the PR group vs controls.
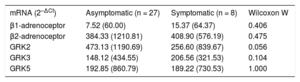
Subgroups of Patients With Pulmonary RegurgitationThe secondary objective was to compare the gene expression of adrenoceptors and GRKs between symptomatic patients with severe PR (NYHA functional class ≥ 2) and asymptomatic patients. There were no statistically significant differences between the 2 groups (Table 3), although symptomatic patients showed nonsignificantly lower expression of GRK2.
Adrenoceptor and GRK Expression According to Symptom Presence
| mRNA (2–ΔCt) | Asymptomatic (n = 27) | Symptomatic (n = 8) | Wilcoxon W |
|---|---|---|---|
| β1-adrenoceptor | 7.52 (60.00) | 15.37 (64.37) | 0.406 |
| β2-adrenoceptor | 384.33 (1210.81) | 408.90 (576.19) | 0.475 |
| GRK2 | 473.13 (1190.69) | 256.60 (839.67) | 0.056 |
| GRK3 | 148.12 (434.55) | 206.56 (321.53) | 0.104 |
| GRK5 | 192.85 (860.79) | 189.22 (730.53) | 1.000 |
GRKs, G protein-coupled receptor kinases; mRNA, messenger RNA.
Gene expression was also analyzed according to the presence of RV dilation and dysfunction, but there were no significant differences in adrenoceptor and GRK expression levels according to these parameters (Table 4 and Table 5).
Adrenoceptor and GRK Expression According to RV Dilatation (RVEDVI > 150mL/m2)
| mRNA (2–ΔCt) | Without significant dilatation (n = 12) | With significant dilatation (n = 23) | Wilcoxon W |
|---|---|---|---|
| β1-adrenoceptor | 8.63 (64.69) | 7.76 (60.00) | 1.000 |
| β2-adrenoceptor | 328.82 (1210.81) | 415.01 (1079.25) | 0.195 |
| GRK2 | 298.75 (834.00) | 468.80 (1190.69) | 0.526 |
| GRK3 | 181.79 (382.39) | 152.60 (303.93) | 0.327 |
| GRK5 | 200.90 (821.51) | 189.91 (730.53) | 0.381 |
GRKs, G protein-coupled receptor kinases; mRNA, messenger RNA; RV, right ventricular; RVEDVI, right ventricular end-diastolic volume index.
Adrenoceptor and GRK Expression According to RV Dysfunction (RVED < 45%)
| mRNA (2–ΔCt) | Without RV dysfunction (n = 22) | With RV dysfunction (n = 13) | Wilcoxon W |
|---|---|---|---|
| β1-adrenoceptor | 8.63 (58.68) | 7.76 (65.46) | 0.724 |
| β2-adrenoceptor | 389.53 (1207.56) | 415.01 (1085.18) | 1.000 |
| GRK2 | 412.85 (974.70) | 468.80 (1190.69) | 0.625 |
| GRK3 | 178.53 (413.47) | 152.60 (434.55) | 0.625 |
| GRK5 | 190.81 (681.35) | 199.29 (873.69) | 0.853 |
GRKs, G protein-coupled receptor kinases; mRNA, messenger RNA; RV, right ventricular; RVED, right ventricular ejection fraction.
In this work, we studied patients with PR from a pathophysiological point of view, surmising that analysis of the molecular changes would allow us to better understand the course of the disease and improve its clinical management. Of the 5 genes studied, a significant decrease was found in the expression of GRK2 and β2-adrenoceptor in the CMLs of patients with PR and with HF vs controls; therefore, PR patients exhibit biochemical data of cardiac damage similar to those observed in patients with advanced HF.
PR continues to be a vitally important sequela in patients who undergo surgical repair of tetralogy of Fallot and pulmonary stenosis and is a highly frequent cause of reoperation. The chronic RV volume overload caused by the presence of PR results in pathophysiological changes that lead to its dilatation and dysfunction, which culminate in arrhythmias, exercise intolerance, HF, and death. The indications for PVR in asymptomatic patients are unclear; although surgery is recommended in certain situations such as progressive RV dilatation/dysfunction,5 with there is no consensus on the optimal timing of surgical intervention. Among the tools that could be used in decision-making, the analysis of molecular parameters that indicate ventricular dysfunction could help in the treatment of these patients.
Because there are alterations in the expression of β-adrenoceptors and GRKs in the CMLs of patients with cardiovascular diseases such as hypertension and HF,14–18,21,22 their use has been proposed as biomarkers of the disease course, particularly for HF. For this reason, it was considered appropriate to study the gene expression of the 2 β-adrenoceptor subtypes (β1 and β2) and the 3 GRK subtypes (GRK2, GRK3, and GRK5) in CMLs to determine if their expression patterns also undergo changes in patients with PR.
For HF, changes have been found in the cardiac expression (gene or protein) of β1-adrenoceptor, GRK2, and GRK5,13,16,23 which vary according to the cause24 or stage25,26 of the disease. Evidence suggests that the changes in the heart are reproduced in CMLs,15,27–30 which would allow CMLs to be used as a mirror of cardiac changes. This parallelism between CMLs and the heart can be explained by considering the possible effects of circulating catecholamines or beta-blocker therapy on the expression of adrenoceptors and GRKs. Indeed, a sustained increase in circulating catecholamines decreases the expression of β-adrenoceptor subtypes but augments the expression of GRKs, whereas beta-blockers have the opposite effect.29,31 However, the changes produced in the heart and the CMLs are not always concordant. Although high concentrations of GRK216,21 and β2-adrenoceptor21 have been described in the myocardium of patients with dilated cardiomyopathy, other results indicated reduced expression levels of GRK2 and β2-adrenoceptor in the CMLs of these same patients and that this decrease is reversed after heart transplant.21
Although the mechanisms underlying the altered expression levels of the adrenoceptors and GRKs in CMLs and their pathophysiological significance32 are poorly understood, various animal models and human studies of cardiovascular disease showed a common pattern of changes between β2-adrenoceptor and GRK2 in each territory,21 a pattern also found here in the CMLs of patients with PR, where both genes decrease. Notably, a similar reduction is observed in the CMLs of patients with HF, a decrease that is reversed after heart transplant,21 which supports their correlation with the disease course. Therefore, the analytical value of the CML expression levels of β2-adrenoceptor and GRK2 does not solely lie in identifying a mirror of cardiac changes, but can also involve their use as a biomarker of CMLs themselves, as proposed by other authors for patients with HF.19
Focusing on the group of patients with severe PR, our GRK2 and β2-adrenoceptor expression pattern in CMLs was similar to that of the HF group but significantly different from that of the control group. Regardless of the origin of this change, this pattern indicates that the degree of ventricular dysfunction and the corresponding molecular changes in the CMLs of patients with PR are similar to those of patients with advanced HF, even though patients with PR are largely asymptomatic. If the reduction in β-adrenoceptors in CMLs observed in different cardiac diseases is related to a low-intensity chronic inflammatory response,32 these findings could indicate that there is already highly advanced RV damage in asymptomatic PR that engenders this inflammatory response. These data could also somewhat explain the absence of a significant improvement in RV functional parameters after PVR and indicate that the repair should be performed earlier. Indeed, Hallbergson et al.,33 analyzing long-term RV remodeling data, observed that, although there was a decrease in RV volumes with a slight reduction in RV ejection fraction in the first 2 years after PVR, the RV volume rose again after 10 years to the pre-PVR values, with a lower RV ejection fraction. Although these findings were related to RV pressure overload and volume due to dysfunction of the implanted bioprosthesis, these changes were also observed when the graft dysfunction was not as significant, indicating that the patients’ right ventricle was highly vulnerable to slight hemodynamic changes, possibly because the ventricular remodeling was already established before the PVR. Similarly, a recent study10 showed that the improvement in RV assessment parameters and oxygen consumption data was greater in patients surgically treated at younger ages, probably because there was less RV remodeling. Taken together, the RV volume and function data obtained using cardiac magnetic resonance imaging are probably insufficient in asymptomatic patients to determine the optimal timing of the PVR and biomarkers are needed to support an earlier surgical intervention. In view of the published results, the concentration of GRK2 does show prognostic value in patients with HF19 and, according to our results, could also be useful in patients with PR. First, it is necessary to show that an early intervention improves prognosis and to fine-tune the surgical technique in order to reduce the number of reinterventions.
No studies have analyzed these biomarkers in right-sided heart disease. A recent study identified altered expression of GRK2 and adrenoceptors in the atrial cardiomyocytes of patients with congenital heart disease who underwent surgical repair involving cardiopulmonary bypass.34 These findings were linked to the vulnerability of the myocardium to catecholamine concentrations in this context and the myocardial damage that this can cause. These results, although interesting, are not comparable to those of this work because our patients were stable.
Evaluation of the group of patients with PR according to their symptomatic status and RV assessment parameters revealed a nonsignificant tendency for lower GRK2 gene expression in symptomatic patients vs asymptomatic patients. The absence of a significant difference can be explained by the small sample size. This finding indicates that symptomatic patients have a higher degree of cardiac damage and that there may be an inverse relationship between the GRK2 concentration and the degree of cardiac damage. However, these differences are not present when patients are compared according to RV damage in terms of dilatation or systolic dysfunction. Thus, although the parameters used for the surgical indication are the RV volume and function values on cardiac magnetic resonance imaging, the cutoff points used to indicate surgery may be too late and the myocardial damage may already be established.
LimitationsAlthough similar to that of previous single-center studies of patients with PR,9 the sample size of the study is small, reducing its statistical power.
Patients with advanced HF may not be comparable with patients with severe PR but represent a population with significant cardiac damage and, in light of the results, its pathophysiology is similar to that of patients with PR.
Various studies have found changes in gene expression in adrenoceptors and their regulating GRKs in different cardiovascular diseases. The present study is the first to show similar gene expression levels of β2-adrenoceptor and GRK2 in patients with PR and advanced HF, although multicenter studies are required to obtain a larger sample size and to determine the prognostic implication of these findings.
CONCLUSIONSThis work examines PR from a novel molecular approach and can aid therapeutic decision-making in these patients.
The main finding of the study is that the gene expression levels of GRK2 and β2-adrenoceptor, as molecular markers of cardiac dysfunction, are altered in patients with severe PR vs controls and are similar to those of patients with advanced HF. These findings should encourage further study of these molecular markers in this population and analysis of their prognostic value and usefulness in the clinical treatment of these patients.
FUNDINGSupport was received from the Instituto de Salud Carlos III, Health Research Fund (FIS PI070509), Spanish Ministry of Economy and Competitiveness (SAF2013-45362-R), and the Valencian Government (GVACOMP07-182 and GVACOMP2009/261).
E. Oliver is a beneficiary of a research grant from the Marie Sklodowska-Curie Actions FP7 COFUND. The CNIC (National Center for Cardiovascular Research) is supported by the Spanish Ministry of Economy, Industry, and Competitiveness and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV-2015-0505).
CONFLICTS OF INTERESTNone declared.
- –
Alterations in the expression of adrenoceptor and GRK genes in CMLs have been found for some cardiovascular diseases and, according to some authors, may have prognostic value in diseases such as HF. Little is known about the molecular alterations in patients with congenital heart disease, particularly in patients with PR. This knowledge could provide information on the molecular changes in these patients and help to determine the optimal timing for PVR to preserve RV function and improve prognosis.
- –
We performed a molecular assessment of patients with PR. Because our results show an altered expression of the β2-adrenoceptor and GRK2 genes similar to that of patients with HF, these biomarkers could be useful for the comprehensive assessment of patients with PR in conjunction with the tools currently in use (clinical assessment and imaging tests). Although further studies are required to confirm these findings and analyze the prognostic value of these biomarkers, this work proposes a new approach for the clinical treatment of patients with PR.