The improvements in cancer detection and therapy have created a new cohort of patients who will experience sufficient survival to develop the cardiac complications of the cancer therapy. Three-dimensional echocardiography has been validated as the ultrasound modality with the best accuracy for the calculation of ejection fraction when compared to magnetic resonance imaging, the current gold standard, making it the tool of choice, when available, for the initial evaluation and follow up of the patient receiving chemotherapy.
If three-dimensional echocardiography is not available, or if the quality of the images is inadequate, the use of ultrasound contrast can be useful for the definition of the endocardial border and identification of the true apex of the heart, enhancing the ability of the interpreter to accurately calculate volumes and ejection fraction.
Two-dimensional strain appears promising as a tool to identify abnormalities in myocardial mechanics very early on during cardiotoxicity, allowing the prediction of later overt systolic dysfunction. This parameter may be useful in the detection of chemotherapy treated patients who could benefit from alternate therapies, thereby decreasing the incidence of cardiotoxicity and its associated morbidity and mortality.
Keywords
The treatment of cancer has progressed in a remarkable way over the last decade. The introduction of targeted therapies has increased the cure and remission rates in some cancers, and in others has converted cancer into a chronic disease. The net result is an emerging cohort of patients who will have sufficient survival to experience the cardiac side effects of the therapies used to treat their neoplasias. Unfortunately, the abundant knowledge that has been collected on the biochemical pathways involved, and as a result targeted in the treatment of cancer, has not been paralleled by an understanding of the cardiac consequences of their modulation. This manuscript will use the treatment of breast cancer as a platform to illustrate the current understanding of the mechanisms of cardiotoxicity, the conventional methods for its evaluation, and the new strategies used for its early detection.
Breast cancer: chemotherapy and cardiotoxicityBreast cancer is the most common cancer in women in the United States. The chance of developing invasive breast cancer during a woman's lifetime is approximately 1 in 7, with a mortality of about 1 in 33.1 As the therapies and survival have improved, more than 2.2 million women are now breast cancer survivors in the United States.2 The prolonged survival resulting from the cancer treatment allows patients to live long enough that cardiac toxicity can be the main determinant of quality of life, and in some cases of premature mortality.3 In fact, for early stage breast cancer, a patient is more likely to die from heart disease than from cancer.4 A number of therapies used in breast cancer are cardiotoxic5 (Table 1).
Table 1. Toxicity of Chemotherapeutic Agents
| Agent | Most frequent toxicity |
| Fluoracil | Myocardial ischemia and infarction |
| Anthracyclines | Cardiomyopathy, myopericarditis, arrhythmias |
| Cisplatin | Hypertension |
| Cyclophosphamide | Heart failure, myopericarditis, arrhythmias |
| Taxanes | Heart failure, ischemia, arrhythmias |
| Methotrexate | Ischemia, arrhythmias |
| Trastuzumab | Heart failure |
| Tamoxifen | Venous thrombosis |
| Radiotherapy | Restrictive heart disease, accelerated atherosclerosis, pericardial effusion |
Anthracycline antibiotics have saved the lives of many cancer victims during the 50years after their discovery. However, a major limitation of their use is the dose-limiting cardiotoxicity. Classically, our understanding of the mechanism of toxicity of anthracyclines has been focused on the role of the reactive oxygen species. More recently, this understanding has been expanded, involving the role of topoisomerase 2. There are two topoisomerase 2 isozymes in mammalian cells: Top2α, and Top2β.6 It is well established that the anti-tumor activity of doxorubicin is mediated by the formation of a Top2α-doxorubicin-DNA ternary complex.7 The high efficacy of doxorubicin is thought to be due to the elevated expression of Top2α in cancer cells. In contrast to Top2α, which is only expressed in proliferating and tumor cells, Top 2β is expressed in the adult heart. Lyu et al recently demonstrated that dexrazoxane antagonizes doxorubicin-induced DNA damage through its interference with Top2β, which could implicate Top2β in doxorubicin cardiotoxicity.8 The cardiotoxicity of anthracyclines, a mainstay of breast cancer treatment, is well known, with a reported overall incidence of symptomatic heart failure between 2.2% and 5.1%.9 The curves drawn by Von Hoff, and from our experience at MD Anderson, appeared flat as long as the patient received a dose lower than 450mg/m2. As a result, physicians felt safe administering doses lower than 450 /m2. However, recent data from an animal model indicates that the toxicity is not as previously thought. Neilan et al developed an acute and a chronic model of toxicity. In the acute model, there was minimal detectable apoptosis at baseline. However, there was evidence of a 75-fold increase in cardiac cell apoptosis just 24h after a single injection of 20mg/kg of doxorubicin.10 The current understanding of anthracycline-induced cardiomopathy involves a dose-dependant loss of cardiac myocytes secondary to apoptosis and necrosis. Following the biomechanical model of heart failure, the ejection fraction (EF) falls as a result of the remodeling of the left ventricle (LV).
Anthracycline-induced cardiomyopathy has been associated with a particularly poor prognosis, with 2-year mortality of up to 60%.11
TrastuzumabThe amplification of the HER2/neu (ErbB2) gene represents a pivotal modification in a subgroup of very aggressive breast cancer. Trastuzumab (Herceptin®) is a humanized monoclonal antibody against the HER2 protein. The development of this antibody as adjuvant therapy for early HER2-positive breast cancers ranks as one of the most satisfying and powerful examples of translational medicine to date. A series of large-scale studies has conclusively shown that trastuzumab can substantially reduce the risk of recurrence and early death in women with HER2-positive breast cancers. Heart failure, a serious side effect of trastuzumab, occurs in up to 4% of patients treated with the antibody. Ten percent of patients have a drop in cardiac function.12
Combination ChemotherapyTrastuzumab increases the cardiotoxicity of anthracyclines. LV dysfunction is noted in 19%-32% of patients in studies administering trastuzumab after anthracycline-based chemotherapy.13 Studies of mutant mouse models have documented an essential role of the ErbB2 gene in the embryonic and postnatal heart. The induction of cardiac stress pathways, by either hemodynamic overload or the cardiotoxicity of anthracyclines, promotes the onset of left ventricular dysfunction in mice that are deficient in ErbB2 protein. The basis for the toxicity is the fundamental role of the ErbB2-ErbB4 heterodimeric receptors in triggering the myocyte-survival pathways required during the activation of acute stress signals. The loss of the survival cues after trastuzumab treatment can lead to irreversible loss of cardiac myocytes during exposure to the anthracyclines. This reasoning is consistent with the clinical finding that trastuzumab also increases the risk of cardiac side effects in patients with existing forms of heart disease in which the cardiac stress signals are presumably already activated.12
Evaluation of cardiac toxicity secondary to chemotherapyLeft ventricular ejection fraction (LVEF) is a robust predictor of outcome, and the variable used historically to evaluate cardiac systolic function at baseline and during chemotherapy. The evaluation of LVEF is commonly done through echocardiography or multiple gated acquisition (MUGA) scan.14
Two-Dimensional EchocardiographyEchocardiography has the advantage of being a non-invasive method that does not involve the use of radiation. In addition to reporting the EF, it provides other information on cardiac morphology, chamber size, and valvular and diastolic function.15 However, the measurement of LVEF presents a number of challenges related to image quality, assumption of left ventricular geometry, load dependency, and expertise.16 As a result, the 95% confidence intervals of measured LVEF are ±11%, failing to detect subtle alterations in LV function. In addition, the inter and intra-observer variability is higher than in MUGA scan (8.8% vs. 6.8% for two-dimensional echocardiography).17
Multiple Gated Acquisition ScanThe measurement of LVEF using MUGA scan has the advantage of lower inter-observer variability (< 5%18) and the lack of geometrical modeling. Disadvantages of MUGA include the exposure to radioactivity and the limited information that can be obtained on cardiac structure and diastolic function.
Magnetic Resonance ImagingCardiac magnetic resonance (CMR) is considered the gold standard for the evaluation of left ventricular volumes, mass, and EF. Its high reproducibility, the lack of geometrical assumptions, and the ease of demarcation of the endocardium from the trabeculation make this technique particularly appealing for the evaluation of LV function. However, its lack of availability and high cost limit its routine use.15
Assessment of cardiotoxicity in the echo labThe sequential monitoring of cardiac function during chemotherapy is of paramount importance to early detection of LV dysfunction. Currently, there are guidelines for monitoring chemotherapy-induced cardiotoxicity in children treated with anthracyclines.19 In the adult population, the American Heart Association recommends close monitoring of cardiac function during anthracycline therapy, although it does not specify the methods, thresholds, or intervals that should be utilized during follow-up.20 For patients treated with anthracyclines, echocardiography has been the preferred method of monitoring cardiac function.21, 22, 23, 24, 25
Definition of CardiotoxicityCardiotoxicity has been defined using various classifications. Recent guidelines suggest that a reduction of LVEF >5% to LVEF <55% with symptoms of heart failure, or an asymptomatic reduction of LVEF of >10% to a LVEF<55%, constitute cardiotoxicity.26
Although LVEF is the most commonly monitored parameter during chemotherapy, its prognostic value in this particular population appears still controversial. In a study of 28 patients with non-Hodgkin lymphoma receiving doxorubicin, Nousiainen et al.27 reported a significant decline of LVEF at low cumulative doses that was predictive of later development of cardiotoxicity. In contrast, in a study of 120 patients with breast cancer followed before, during, and 3years after treatment with epirubicin, monitoring of LVEF did not seem to correlate with later development of cardiotoxicity.28 However, Alexander et al.29 demonstrated its usefulness in the sequential evaluation of LVEF in clinical practice.
Improved Measurements of Left Ventricular Ejection Fraction With Novel TechnologyNewer technology has emerged that allows for an improvement in the accuracy of calculating EF.
Contrast EchocardiographyContrast echocardiography defines the endocardial border better than unenhanced echocardiography and, compared with unenhanced echocardiography in numerous single center and multicenter studies, shows better agreement and reduction in intra-observer and inter-observer variabilities in measured LV volumes and LVEF, with the use of current reference standards. The American Society of Echocardiography guidelines state that the understimation of cardiac volumes by echocardiography can be nearly resolved when contrast agents are used.30
Three-Dimensional EchocardiographyThe calculation of EF using 2D echocardiography has important limitations, based on geometrical models that do not take into consideration the architecture of the sick heart, and that are strongly affected by foreshortening. Real time three-dimensional echocardiography (RT3DE) has emerged as a solution to these problems. The ability to capture a full volume acquisition of the LV allows for accurate identification of the true apex of the heart. An algorithm based on the detection of the endocardial border then allows for direct quantification of LV volumes, without multiplane tracing or geometric modeling.
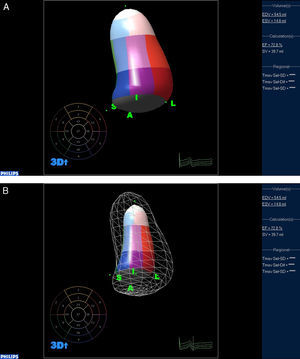
Jacobs et al. compared two-dimensional and three-dimensional echocardiography against CMR imaging for their ability to accurately calculate the end diastolic volume (EDV), end systolic volume (ESV), and EF. The RT3DE measurements of LV volumes correlated highly with CMR imaging values (r=0.96, r=0.97 and r=0.93 for EDV, ESV, and EF, respectively)31 (Figure 1). The LV volume assessment and calculation of EF by RT3DE is a rapid, accurate and reproducible method, superior to conventional 2D methods. The small negative biases of the calculation of volumes and EF, as compared to CMR, should be reduced as we gain experience with this new technique, and as we learn to trace the endocardium underneath the trabeculations, and not on top of them.32
Figure 1. Calculation of left ventricular volumes and ejection fraction using three-dimensional echocardiography. A: End diastolic frame. B: End systolic frame.
Contrast-Enhanced Three-Dimensional EchocardiographyMore recently, contrast has been used to enhance RT3DE images. Contrast enhancement was found not only to improve the accuracy and reproducibility of LV volume measurements in patients with poor image quality, but also to enhance the assessment of regional wall motion from RT3DE datasets. With the use of selective dual triggering to minimize bubble destruction by ultrasound energy, contrast enhancement increased the accuracy of RT3DE-based analysis of regional LV function against CMR reference, and improved its reproducibility to levels similar to those noted in patients with optimal imaging quality.33
Implications of a More Accurate Ejection FractionThe improved accuracy and reproducibility of RT3DE-based LV volumes and LVEF measurements are of vital importance in the patient receiving chemotherapy, since clinical decision making relies completely on this measurement. In the study mentioned above by Jacobs, there was evidence of a wider limit of agreement for EDV, ESV and EF for two-dimensional transthoracic echocardiography (2DTTE) (29%, 24% and 9.5%, respectively) compared to RT3DE (17%, 6% and 6.4%).31 This means that when using 2DTTE, the EF can potentially be miscalculated by 9.5 points.
Anthracyclines are discontinued if patients have a symptomatic drop of more than 5% of their EF, to below 55%, or an asymptomatic drop of more than 10% of their initial EF. Miscalculation of the EF by 2DTTE can lead to a decision by the oncologist to stop the anthracycline-based regimen due to concern for toxicity, in a patient that actually doesn’t have it, where the mistake in the calculation of EF is solely the result of the inherent limitations of the technology used.
Diastole and CardiotoxicityIn chemotherapy-induced cardiotoxicity as in other cardiac conditions (such as ischemic cardiomyopathy34, 35, 36, 37), alterations in diastolic dysfunction may precede the systolic dysfunction. The abnormalities of the diastolic parameters seem to represent an early sign of LV dysfunction in patients treated with chemotherapy.38, 39, 40 In a study of 26 patients with acute leukemia treated with 2-6 cycles of anthracycline-based chemotherapy, the changes in diastolic function developed very early on, after the initiation of chemotherapy, with significant reduction in the E/A ration and prolongation of both deceleration and of isovolumetric relaxation time before LVEF decreased.41 Stoddard et al.42 prospectively evaluated 26 patients before beginning chemotherapy (doxorubicin) and 3weeks after cumulative doses. He observed prolongation of the isovolumetric relaxation time preceding a significant decrease in LVEF. These studies reinforce the predictive value of the diastolic indices for the development of subsequent cardiotoxicity.
Stress Echocardiography and CardiotoxicityExercise and pharmacologic stress testing has been studied as a way to unmask subclinical LV dysfunction. In 31 cancer patients studied before, during, and 6 months after chemotherapy, low-dose dobutamine did not provide additional value for the early detection of cardiotoxicity.43, 44 However, in 26 patients treated with high-dose anthracyclines and without symptoms of cardiac dysfunction, high-dose dobutamine revealed an alteration of the fractional shortening and the transmitral E/A ratio.45 Exercise echocardiography can unmask subclinical cardiac dysfunction, as demonstrated by Jarfelt et al.46 in 23 young adults. These patients were acute lymphoblastic leukemia survivors who had received anthracyclines before the onset of puberty and were followed for a median of 21years after remission. Ten of the 23 patients presented with reduced LVEF at stress; reduced LVEF was not observed in any of the controls.
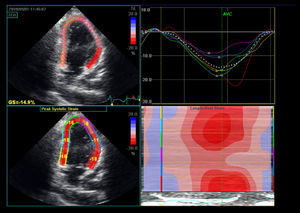
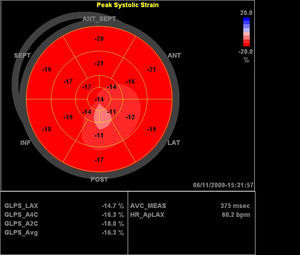
Two-Dimensional Based StrainTwo-dimensional strain (2DS) is an automated quantitative technique for the measurement of global long-axis function from gray-scale images. Longitudinal tissue deformation is evaluated by frame-by-frame tracking of individual speckles throughout the cardiac cycle (Figure 2, Figure 3). Global longitudinal strain (GLS) is calculated from the mean of 18 cardiac segments. The 2DS technique is more robust than tissue-Doppler-derived strain, does not suffer from angle dependency, and is easier to calculate. The following studies provide evidence for the superiority of 2D-based strain as compared to EF for the evaluation of the chemotherapy patient.
1. Strain Versus Ejection Fraction in the Prognosis of All Cause Mortality in the General Population. Stanton et al. recently published a study comparing GLS against EF for the prediction of all cause mortality in the general population. He concludes that GLS is superior to either EF or wall motion score index as a predictor of outcome and may become the optimal method of assessment of global LV function.16
2. Strain as a New Prognosticator in Acute Heart Failure. The study published by Cho et al. concludes that strain is a powerful predictor of cardiac events and appears to be a better parameter than EF in the evaluation of patients with acute heart failure.47
3. Reliability of the Technique. Marwick et al. published a brief report on normal GLS values and their reliability, evaluating 242 patients. Inter-observer variability (comparison between sites) was measured in 253 segments. The mean difference in measurements was 0.24 percentage points. A total of 38 patients underwent successive tests within 1 h; the test-retest variability showed no systematic bias, and 95% confidence intervals were between -9.6% and +9.7%.48
4. Strain Rate and Early Detection of Cardiotoxicity. Marwick sought to determine whether changes in tissue deformation, assessed by myocardial strain and strain rate [SR], were able to identify LV dysfunction earlier than conventional echocardiographic measures in patients treated with trastuzumab. Study of 152 sequential echocardiograms in 35 female patients showed significant reductions seen in tissue Doppler imaging strain rate (P<.05), 2D-SR (P<.001) and 2D radial SR (P <.001). Of the 18 patients with reduced longitudinal SR, 3 had a concurrent reduction in EF ≥10% and another 2 showed a reduction over 20 months follow-up.49
5. Global Longitudinal Strain and Early Detection of Cardiotoxicity. In this second study, our group, in collaboration with the echocardiography laboratory at Massachusetts General Hospital and others, sought to evaluate whether sensitive echocardiographic measurements and biomarkers could predict later cardiac dysfunction in 43 chemotherapy-treated patients. Measurements included LVEF peak systolic myocardial longitudinal and radial strain, echocardiographic markers of diastolic function, N-terminal pro-brain natriuretic peptide (NT-proBNP), and cardiac troponin I (cTnI). Nine patients (21%) developed cardiotoxicity (1 at 3 months and 8 at 6 months). The decrease in longitudinal strain from baseline to 3 months and a detectable troponin at 3 months were independent predictors of the development of cardiotoxicity at 6 months. Left ventricular EF, parameters of diastolic function, and NT-proBNP did not predict cardiotoxicity.50
6. Radial Strain. Recently, Jurcutt et al.51 demonstrated that myocardial deformation parameters, which included longitudinal and radial strain and strain rate, allowed detecting subtle alterations in longitudinal and radial LV function following 6 cycles of pegylated lipososomal doxorubicin in 16 elderly women with breast cancer. The LV dimensions, EF, and systolic myocardial velocity did not change during the follow-up.
Figure 2. Two-dimensional-based strain of a patient with anthracycline-induced cardiomyopathy. The mid and apical segments of the anterior wall exhibit abnormal regional strain.
Figure 3. Polar map of the patient with anthracycline-induced cardiomyopathy referenced in Figure 2 .
The author postulated that an abnormality in radial strain could be the earliest manifestation of toxicity expressed in the population studied.
Conflicts of interestDr. Plana has received clinical research support from General Electric and participated in educational activities sponsored by this company.
Corresponding author: Section of Imaging, Department of Cardiovascular Medicine, The Cleveland Clinic, 9500 Euclid Avenue / J 1-5, Cleveland, Ohio 44195, United States. planaj@ccf.org