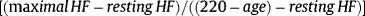Heart rate (HR) is a reflection of the balance between the sympathetic and parasympathetic divisions of the autonomic nervous system, and chronotropism is the effect of any substance or situation on this parameter.1,2 Chronotropic response can thus be defined as the ability of HR to adapt to the activity level or metabolic demand of the body.3 In healthy individuals, oxygen uptake increases by up to 4-fold during maximal aerobic exercise. More than half of this effect is caused by increased HR, followed—in order of magnitude—by higher peripheral arteriovenous oxygen difference and stroke volume.1 Increased HR during physical exercise is considered an essential physiologic reflex and is the main cause of increased cardiac output during +exertion, particularly in the middle-to-final phases. Consequently, chronotropic incompetence is defined as the inability to increase HR in response to greater demand or activity, possibly contributing to an individual's exercise intolerance.1,2
The literature on cardiovascular conditions and on exercise physiology contains various definitions of chronotropic incompetence, with the ultimate aim of establishing an objective criterion. However, these disparate approaches to defining this concept have probably hindered scientific evidence to date, due to a lack of consistency.1 For instance, Coman etal4 studied around 1500 patients with a pacemaker. When 5different definitions of chronotropic incompetence were applied in that study, the prevalence varied between 34% and 87%. Chronotropic incompetence has traditionally been diagnosed on the basis of an inability to achieve a theoretical maximal HR percentage during an incremental stress test. The limits most commonly used are 85% or 80% and, less often, 70%. These estimates are based on the theoretical maximal HR formula of “220 – age” described in the 1960s by Astrand et al.5 This formula is widely used in clinical practice, despite its known limitations due to large interindividual variability, particularly in patients with ischemic heart disease and/or treated with beta-blockers.1,2 Another way to define chronotropic incompetence that is more frequently recommended and used in the current literature is to modify the equation to include resting HR and its difference with the maximal HR obtained, a parameter known as HR reserve. According to this formula, chronotropic incompetence is defined as an inability to achieve 80% of HR reserve on maximal stress testing. Currently, the most widely used formula is the following1,2:
Before concluding that a patient has chronotropic insufficiency, it is important to ensure that the stress test has been truly maximal. Currently, the most objective way to measure the level of exertion during exercise is through the respiratory exchange ratio (RER) during cardiopulmonary stress testing. A peak RER <1.05 on maximal exercise is considered to indicate submaximal effort and, therefore, diagnosis of chronotropic incompetence should be made cautiously in these cases.1,2
The current gold standard to measure or establish functional capacity in healthy individuals or in patients with heart failure (HF) or other conditions is cardiopulmonary stress testing.6 Chronotropic incompetence is common among patients with cardiovascular diseases. According to current scientific evidence, exercise intolerance is related to adverse effects on quality of life, and it is also an independent predictive factor of adverse cardiovascular events and all-cause mortality in both asymptomatic individuals and in patients with ischemic heart disease and with HF.1,2
In a recent single-center study published in Revista Española de Cardiología by Palau et al., 7 the authors included 133 stable outpatients with HF and preserved ejection fraction (HFpEF) to evaluate HR response on maximal cardiopulmonary stress testing. The chronotropic index was calculated using the above formula. Patients were classified into 2 subgroups according to whether they were in sinus rhythm or atrial fibrillation (AF). The primary endpoint of the study was worsening HF, defined as the need for intravenous diuretic administration. Secondary endpoints, such as all-cause mortality or hospitalization due to HF, were also established. Mean follow-up was 2.4 years, and patients in AF had baseline characteristics indicating more advanced HF—mainly higher concentrations of NT-ProBNP (325 vs 10 095pg/mL; P <.001) and greater use of furosemide (41.5% vs 67.7%; P=.002). However, there were no differences in New York Heart Association (NYHA) class, maximal oxygen uptake, resting HR, or chronotropic index between the 2 subgroups or in beta-blocker treatment (90% in both subgroups). The primary endpoint showed no significant association between worsening HF events or all-cause mortality and the chronotropic index in the overall study population.
Nevertheless, when the chronotropic index was analyzed using a median split (< 0.4 vs ≥ 0.4), a significant difference was seen in the rates of worsening HF below 0.4 in patients in sinus rhythm, whereas patients in AF exhibited a nonsignificant trend in the opposite direction. Last, the adjusted multivariate analysis showed a prognostic effect of the chronotropic index on the primary endpoint of the study. In other words, in patients in sinus rhythm, a lower chronotropic index was seen to be associated with a higher risk of HF events, whereas the opposite was found in patients in AF: higher HR rates were associated with a higher need for intravenous diuretics. These associations were linear and significant. This effect was also seen in hospitalizations due to HF, but was not statistically significant for mortality. The authors conclude that chronotropic response was associated with a higher risk of worsening HF events depending on heart rhythm (sinus rhythm vs AF) in stable outpatients with HFpEF.
A common clinical characteristic of all patients with HF is a significant reduction in physical capacity, with a 15% to 40% decrease in maximal oxygen uptake, compared with age-matched controls. This occurs in both HFpEF, as seen in the present article, and with reduced EF, for which there is more evidence.1,2,8 From a pathophysiologic point of view, reduced cardiac output in patients with HF has usually been attributed to lower stroke volume due to a structural cardiac condition, such as systolic or diastolic dysfunction, valve disease, etc. This reduction in stroke volume increases HR to compensate for the lower cardiac output during exercise, thus explaining the higher resting HR values in patients without HF,9 mainly due to pathophysiologic mechanisms of the autonomic nervous system, in which sympathetic activity predominates over parasympathetic activity. Consistent with this, it has been established that, as HF severity increases, a higher amount of norepinephrine is needed to raise HR; this mechanism has long been associated with a decreased density and desensitization of beta receptors in circulating catecholamine concentrations.10,11
Another essential consideration in this population is medication, as investigated in previous studies by this group.12 A nonnegligible number of cardiovascular treatments have a direct effect on HR. The most widely known example is beta-blockers, but other drugs should be considered, for instance, ivabradine, digoxin, nondihydropyridine calcium blockers, and various antiarrhythmic agents. Paradoxically, several studies13,14 have found no difference between patients with HF treated with beta-blockers and those not receiving these drugs; ie, the number of cases of chronotropic incompetence was similar, regardless of the use of these drugs. It has been postulated that this may be due to the study population, because beta-blockers are less effective in advanced stages of the disease.2 This phenomenon could be explained by a lower tolerance to these agents or a lower quotient between chronotropic response and catecholamine concentration.
There is some controversy regarding the role of beta-blockers in HF, as these drugs have been shown to have a prognostic benefit in HF with reduced ejection fraction but are also a proven cause of chronotropic incompetence and worsening HF events. A meta-analysis of various clinical trials with beta-blockers in HF showed that the effect of decreased mortality due to these drugs was related to the decrease in resting HR, whereas a lower maximal HR was in turn associated with worse prognosis,15 which would explain this controversy. Irrespective of these considerations, the current literature has not established a cutoff point to define chronotropic failure in patients with HF receiving negative chronotropic drugs. Although the chronotropic index limit of 0.4 used in that study seems to be an arbitrary choice (based on the median of the study population), this limit could provide evidence for defining chronotropic incompetence in patients with HFpEF, as it was associated with events in nearly 90% of patients in sinus rhythm who were receiving this treatment.
In view of the above, it is truly challenging to establish the prevalence of chronotropic incompetence in patients with HF, and the literature reports wide variability, ranging from 25% to 70%, depending on the definition.1 Using the traditional formula of theoretical maximal HR (220 − age), the prevalence ranges from 42% to 61%, whereas the proposed formula, which includes resting HR —[(maximal HR − resting HR) /(220 − age) − resting HR)]— yields an even higher prevalence but with a narrower range: from 72% to 84% of patients with HF.2 Chronotropic incompetence in HF has been directly associated with mortality, hospitalization, and impaired quality of life in patients with this syndrome in studies since the 1980s,1,2 although the causality is not clear. However, we believe that chronotropism is rarely assessed in patients in daily clinical practice, at least in our setting. This may be partly due to the multiple definitions of the condition, the potential bias resulting from the confounding factors of aging and medication when identifying it, and/or the need to perform formal stress tests to reach a definitive diagnosis.
The authors should be congratulated for their determination in conducting a study in a cohort of patients with HFpEF, as these studies are often difficult because the population is extremely heterogeneous and because there has been no evidence for specific treatments until very recently (with the latest publications of clinical trials on sodium-glucose cotransporter 2 inhibitors). It is worth noting that the primary endpoint chosen was HF decompensation (need for intravenous diuretics), given that the most frequent clinical manifestation of chronotropic failure leads to a stronger focus on quality of life, functional capacity, or patient-reported symptoms. Nevertheless, it is an ambitious endpoint that ultimately produced favorable results, consistent with prior evidence on chronotropic incompetence and adverse events in patients with HFpEF.
This article revisits a classic concept rarely used in routine clinical practice: chronotropic response assessment in patients with HFpEF. In our opinion, it would be key to attempt to classify various phenotypes within this syndrome, which could be an alternative therapeutic target to the treatment of congestion. In this regard, cardiopulmonary stress testing should be considered in all these patients, unless contraindicated, to evaluate physical capacity and to investigate the main cause of the symptoms. In patients in sinus rhythm treated with beta-blockers who exhibit a poor chronotropic response, it may be possible to lower the dose or to discontinue negative chronotropic therapy, which is sometimes started with no clear scientific evidence or as antihypertensive agents and maintained due to therapeutic inertia. This has already been proposed by these authors in previous papers.12 Conversely, in patients in AF, this treatment could be considered in patients with a high chronotropic index (despite the lack of benefit of beta-blockers in general) in order to control HR.
The chronotropic index may be a useful to add clarity to the somewhat catchall diagnosis of HFpEF, which varies widely among patients. Previous evidence plus the findings reported in this article increasingly emphasize an individualized approach to identifying patients with HF, in whom heart rhythm (sinus vs AF) and stress testing (including oxygen uptake) may lead to specific decisions and prevent physicians from treating all patients with HFpEF in the same way. The only intervention that would likely be beneficial for each and every patient with HF is participation in a cardiac rehabilitation program. Once again, however, the program would have to be adapted to each patient's unique characteristics. Ample evidence shows that maximal HR can be increased in patients with HF treated by physical exercise training programs, although the mechanism that increases peak HR is not completely clear. A meta-analysis of 35 randomized studies of patients with HF undergoing cardiac rehabilitation found that HR was increased compared with prerehabilitation values.2,16
Despite the clear association between chronotropic incompetence and adverse events in patients with HF, causality is not well established. It is unclear whether chronotropic failure is a cause or a consequence of these patients’ reduced functional capacity.2 Determining causality would be pivotal, because if chronotropic incompetence is the cause, then it would be a useful therapeutic target, whether through the discontinuation or reduction of bradycardia-inducing drugs or through the implantation of implantable devices with activity sensors.
This article has attempted to delve deeper into the definition, mechanisms, diagnosis, and treatment of chronotropic incompetence, with special emphasis on its key role in HF. Chronotropic incompetence is common and can be diagnosed by widely available objective methods, without huge financial investment. It is also potentially treatable, and the assessment and approach taken to the condition could probably lead to significant improvement in exercise tolerance and quality of life, as well as reduce hospitalizations and even mortality. Widespread use of cardiopulmonary stress testing is essential in daily clinical practice, and the importance of cardiac rehabilitation programs should be emphasized.
Last, we would like to thank Palau et al. for their article and for the contributions made to patients with HFpEF, as they focus their efforts on improving phenotype definitions and moving toward individualized precision medicine.
FUNDINGThe authors state that this project has not received any outside funding.
CONFLICTS OF INTERESTThe authors declare that they have no conflicts of interest related to this editorial.