In 2009, 2343 catheter ablation procedures were performed in Spain for focal atrial tachycardia or atrial flutter (typical and atypical), with a yearly growth rate of 8%, indicating the clinical importance of these arrhythmias. The classic categorization of atrial tachycardia and atrial flutter based on rate and morphological criteria has become almost irrelevant at a time when clinical electrophysiology may lead to curative intervention based on a definition of the mechanism, making it necessary to bring laboratory experience closer to clinical practice. In this review we outline our present understanding of atrial tachycardia mechanisms, both focal and macroreentrant, and attempt to establish the conceptual links with classic concepts that may help the clinician to make a differential diagnosis and establish therapeutic indications, including that of an electrophysiologic study. Some of the concepts may seem complex, but we thought it important to provide an overview of the electrophysiological methods that may eventually lead to the description of the anatomic bases of the arrhythmias; currently, these are easier to understand thanks to the virtual anatomic casts built using computerized navigation systems.
Keywords
.
IntroductionSince the 1970s, knowledge of the mechanisms and pathologic substrates of atrial tachycardias has evolved within the setting of clinical electrophysiology. Electrocardiogram (ECG) analysis had already led to a classification based on pathogenic hypotheses, but atrial activation mapping and the study of stimulation responses have allowed a more accurate definition of the mechanisms and their anatomic substrates, enabling a “curative” approach using catheter ablation or surgery in many cases. On the other hand, cardiac surgery, particularly congenital heart disease surgery, and more recently some radiofrequency ablation treatments, are showing striking arrhythmogenic potential that may lead to a new generation of iatrogenic tachycardias that could be problematic in the future.
In this review, we provide a perspective on atrial tachycardias in a clinical setting, linking clinical manifestations to electrophysiological mechanisms and pathogenic substrates to establish prognoses and make rational therapeutic decisions.
Electrocardiographic diagnosis Focal Atrial TachycardiaFocal atrial tachycardia (FAT) is defined as a rapid atrial rhythm, regular, not originating from the sinus node, with stable P-wave morphology on ECG and that only requires atrial structures to be maintained (Figure 1).1 Alternating P waves and a stable baseline on ECG indicate that the tachycardia is the focal mechanism with silent periods between focal discharges (Figure 2), but there are exceptions to this rule.
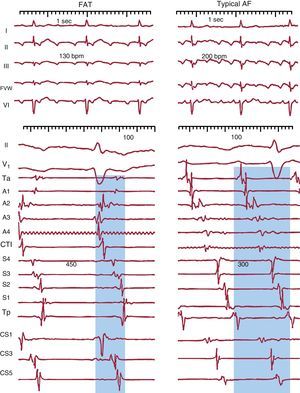
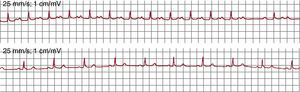
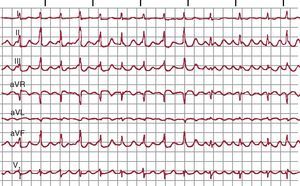
Figure 1. Electrocardiogram and endocardial recordings of focal atrial tachycardia and typical atrial flutter in a single patient. The lower panels show bipolar endocardial recordings of the anterior right atrium (Ta-A4 from top to bottom), the cavotricuspid isthmus, septal right atrium from bottom to top (S4-Tp) and the proximal to distal coronary sinus (CS1-CS5). Blue shading shows the portion of the tachycardia cycle covered by the activation; <50% in focal atrial tachycardia and 100% in atrial flutter, even when only taking into account recordings of the right atrium alone. AF, atrial flutter; CS: coronary sinus; CTI, cavotricuspid isthmus; FAT, focal atrial tachycardia; FVW: flow velocity waveform.
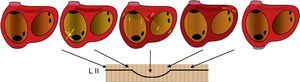
Figure 2. Schematic representation of the atrial activation sequence in focal atrial tachycardia originating from the lower portion of the crista terminalis in relation to the P wave in lead II. Arrows indicate the activation sequence. Flat baselines correspond to silent periods. L, lead.
Atrial FlutterThe name atrial flutter (AF) has been given to a very high frequency (240-350 bpm) atrial tachycardia with atrial waves that produce a continuous oscillation without a flat baseline (Figure 1).1 This pattern would indicate continuous activity during the entire atrial activation cycle, as in the case of typical, or common, AF (Figure 3), but there may be a continuous wave pattern in rapid FAT in which P-wave duration is very close to the cycle length (CL).
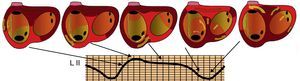
Figure 3. Schematic representation of the atrial activation sequence in typical atrial flutter in relation to the atrial flutter wave in lead II. In this case, there are silent periods because of constant activation. The flatter portion of the atrial flutter wave corresponds to activation of the cavotricuspid isthmus which has little myocardial mass. L, lead.
The classic ECG-based diagnoses of tachycardia and AF are of little importance today because treatment is based on the direct management of the trigger mechanism, whether FAT or macroreentrant atrial tachycardia (MAT), and although some very typical patterns help to locate a FAT focus or to show the typical AF mechanism, in many cases only the electrophysiological study will allow accurate characterization.
Electrophysiological mechanisms Focal Atrial TachycardiaAs shown in Figure 2, Figure 4, FAT is characterized by radial and centrifugal atrial activation from the point of origin, which can be located anywhere in the atria, pulmonary veins, superior vena cava or coronary sinus.2 This definition does not address the issue of whether the focal discharge mechanism is a rapid depolarization of action potential during phase 4, afterdepolarizations (oscillations of the membrane), or a very small diameter reentry circuit (microreentrant). In some cases the electrophysiological mechanism can be deduced from the response to stimulation or drugs,3 but in practical terms defining the discharge mechanism of the initiating focus has become almost irrelevant.
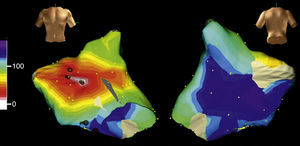
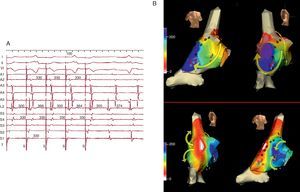
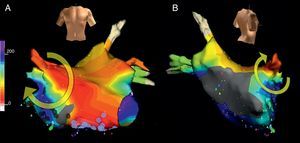
Figure 4. Electroanatomical mapping (Navx® system) of focal atrial tachycardia originating in the paraseptal left atrium. The image of the torso indicates the direction of view (left: anterior; right: posterior with caudal angulation). The time scale on the left marks the beginning of activation (white) and end (violet). Note the uniform centrifugal spread (white → yellow → green → blue → violet) which converges in the posterior left atrium.
The ECG recordings of FAT show a regular P-wave morphology that depends on the source location,2 a rate that can be very close to sinus rhythm, around 100 bpm, or very high, more than 200 bpm (exceptionally up to 300 bpm). When the rate is slow, diagnosis is only possible because of the different morphology (axis, configuration) of the P wave in relation to sinus rhythm (Figure 5). Although the CL or P-P interval of the FAT is usually regular, when its beginning and end are recorded an acceleration during the initial 4 to 5 cycles and prolonged or oscillating cycles preceding spontaneous termination is relatively common. In some cases of stable sustained FAT, oscillations in baseline cycle length may be recorded (Figure 6A). In the absence of other abnormalities, FAT involves radial and rapid atrial activation, but when there are areas of conduction block, activation becomes asymmetric and may resemble “circular” activation as in MAT (Figure 6B).
Figure 5. Lead II electrocardiogram: focal atrial tachycardia (upper) at 100 bpm and sinus rhythm (lower) in a single patient. This confirms the atrial mechanism of atrial focal tachycardia because it is interrupted by a blocked P wave. Note the difference in P wave morphology.
Figure 6. A, electrocardiogram and right atrial endocardial recording of focal atrial tachycardia in a patient undergoing intervention for tetralogy of Fallot. Recordings A1→A5 are of the anterior right atrium from top to bottom, S5→S1 septal right atrium from bottom to top and L2 the focus of tachycardia in mid-upper lateral right atrium (see B). The left part of the recording shows stimulation (S) of the right atrial roof (T) at a cycle length of 300ms with capture of all recording points except for L2, which shows independent cycle oscillations with and without stimulation. Note that L2 electrogram is delayed during stimulation and very early after stimulation. Time scale in milliseconds. B, Electroanatomic mapping of scar macroreentrant atrial tachycardia and focal atrial tachycardia in the same patient of recording A. The dark spots mark the position of the scar (double electrograms of conduction block). Activation in macroreentrant atrial tachycardia (above) goes down the anterior right atrium (yellow arrows: orange→yellow→green→blue) and turn toward the posterior wall between the lower end of the scar and the inferior vena cava. The superior vena cava appears to be included in the central obstacle of the circuit. Red and blue points mark the isthmus where radiofrequency application interrupts the tachycardia. Activation of focal atrial tachycardia (lower) begins next to the scar (white) but does not spread to the anterior right atrium due to the effect of the scar (arrows) and the anterior right atrium undergoes very delayed activation (blue). This focal atrial tachycardia was eliminated with applications on the area of origin (white).
Macroreentrant Atrial TachycardiaIn MAT, atrial activation occurs in a continuous, uninterrupted manner because of a wavefront rotating around an obstacle formed by anatomical structures (venous or valvular orifices), scars, or areas of functional (anisotropic) block (Figure 7). The diameter of the circuit should be ≥2cm to correctly map it using current electrophysiological techniques; smaller circuits would be difficult to distinguish from focal mechanisms.1
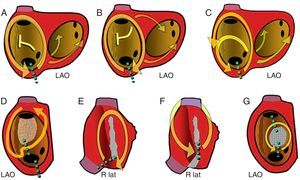
Figure 7. Diagrams of macroreentrant atrial tachycardia of the right atrium observed in clinical practice. The left anterior oblique view of the right atrium shows the orifices of the cavae and the coronary sinus and the crista terminalis (vertical strip). The arrows indicate the direction of activation. The green dotted lines mark the critical isthmus of each circuit, the target of ablation. A, Typical atrial flutter; B, Reverse typical atrial flutter; C, Typical atrial flutter with conduction through the crista terminalis; D, Macroreentrant atrial tachycardia with a nonsurgical low-voltage unexcitable area; E, Scar macroreentrant atrial tachycardia without including the superior vena cava; F, Macroreentrant atrial tachycardia with an isthmus between two scars including the superior vena cava; G, Macroreentrant atrial tachycardia revolving around a septal patch. LAO, left anterior oblique; R lat, right laterial.
Typical AF, also called common AF due to its frequency of presentation, is distinguished from all MATs by its dependence on the anatomical structure of the right atrium (RA) and its clear identification on ECG,4 but other MATs are more difficult to classify, especially in patients with previous cardiac surgery with extensive atriotomy or who have undergone extensive ablation of the left atrium (LA) myocardium for the treatment of atrial fibrillation.5 The ECG of MAT may resemble a FAT with P-waves separated by isoelectric baselines at frequencies ≤200 bpm when there are areas of very slow conduction in the reentry circuit; these generate very low amplitude potentials that are not detected on the surface ECG, but are detected on intracardiac recordings (Figure 8). On the other hand, a FAT can produce a continuous oscillation pattern on ECG if the CL is sufficiently short to approach the duration of atrial depolarization (Figure 9).
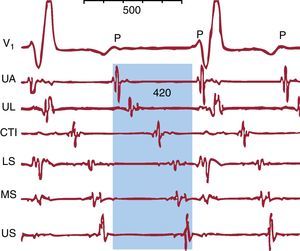
Figure 8. Macroreentrant atrial tachycardia with an electrocardiographic pattern of atrial tachycardia. V1 shows regular low-voltage P waves. The endocardial recordings of the upper anterior, upper lateral, lower septal, mid-septal, upper septal right atrium and cavotricuspid isthmus show low-voltage electrical activity throughout the macroreentrant atrial tachycardia cycle (shaded blue) that is not recorded on the electocardiogram. CTI, cavotricuspid isthmus; LS, lower septal; MS, mid-septal; UA, upper anterior; UL, upper lateral; US, upper septal.
Figure 9. Focal atrial tachycardia (origin in the pulmonary vein) at 300 bpm with a continuous wave pattern on the electocardiogram.
Typical Atrial FlutterTypical AF is the most common MAT and can usually be identified by a very characteristic ECG pattern in the inferior leads which, although often described as a negative wave, is actually a complex wave with a slowly falling phase, followed by a small rapid fall (the negative wave) and a rapid rise that ends in a positive deflection to link with the slow fall during the next cycle (Figure 1, Figure 3, Figure 10).
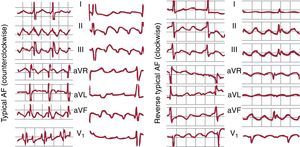
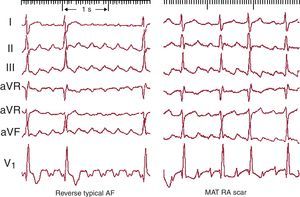
Figure 10. Left, electrocardiogram in 2 cases of typical atrial flutter (counterclockwise) with typical multiphase waves in leads II, III and aVF and with biphasic positive deflections in V1. Right, 2 cases of reverse typical atrial flutter (clockwise) with positive waves in leads II, III and aVF and the typical W pattern in V1 (see text for further explanations). AF, atrial flutter.
The high incidence of typical AF and its replicable pattern on ECG are due to the strong anatomical configuration of the circuit, which drives circular activation around the tricuspid annulus. The activation wavefront goes up the septal RA, turns across the roof, goes down the anterolateral RA, and finally passes between the tricuspid annulus and inferior vena cava to reach the septal RA again. The circuit is stable due to a large obstacle in the posterior RA formed by the orifices of the superior and inferior vena cava linked by the crista terminalis (Figure 7, Figure 10).
RA mapping during a typical AF shows a line of conduction block in the posterolateral wall between the orifices of the vena cavae, which forces the activation front to revolve around them. It is currently accepted that this line of block is due to the anisotropy of the crista terminalis, which due to its vertical orientation facilitates conduction from the sinus node to the inferior RA but blocks transversal conduction in the posterior wall.6, 7 Spach et al. studied canine RA pectinate muscle in which the myocytes are arranged in parallel. They showed that longitudinal conduction in the myocardial bundle was much faster than in the transverse direction, and that this difference was associated with intercellular resistance in both directions8 and the arrangement of low-resistance intercellular junctions (gap junctions) at the ends of the myocytes.9 Transversal conduction block in the crista terminalis10, 11 and the thickness of the ridge itself12 has been associated with the clinical incidence of typical AF.
In 90% of cases, the rotation of the activation front in typical AF is counterclockwise in the left anterior oblique view (going down the anterior RA and up the septal RA). This preference could be because it is easier for a conduction block to form in the lower septal RA where the myocardium is thin and has an irregular alignment. If an extrasystole is blocked at this level, the activation front going down the anterior wall could reenter the septal RA through the cavotricuspid isthmus (CTI), thus initiating counterclockwise rotation. Reverse typical AF (clockwise) travels the same circuit, going down the septal RA then up the anterolateral RA, with the same line of block in the posterolateral RA and the same lower turning point through the CTI (Figure 7, Figure 10). The ECG pattern differs from that of counterclockwise AF with inscription on the lower wall of M-shaped sawtooth positive deflections and negative wide W-shaped sawtooth waves in V1 (Figure 10).
The lower turning point of the circuit is the CTI, a relatively narrow segment of myocardium between the inferior vena cava and the tricuspid annulus, which due to its size and ease of access is accepted as the ideal target for catheter ablation. The terminal ramifications of the crista terminalis insert into the tricuspid ring in the CTI with an irregular alignment of the fibers,13 which moderately slows conduction at this level due to local anisotropy.14
Variations in this typical AF circuit have been described in which the activation wavefront crosses the crista terminalis at some level, while turning in front of the superior vena cava,15 but these variations have no clinical or therapeutic importance (Figure 9).
Atypical FlutterThe term atypical AF includes various ECG patterns and tachycardia mechanisms.5, 16 The current literature also includes within this term MAT with well-defined P-waves, flat baselines, and a frequency ≤200 bpm as well as rapid tachycardias without a stable baseline that do not present the morphology characteristic of typical AF.17, 18, 19, 20 To avoid confusion, we describe MAT circuits according to their anatomical substrate based on the fact that electrophysiological studies alone allow us to define the mechanisms by which an effective therapeutic approach can be guided.
Macroreentrant Right Atrial TachycardiaAtriotomy, which is widely used for the correction of atrial or ventricular septal defect, and myxoma resection or tricuspid valve repair may create a structure that can act as a central obstacle in MAT, and is usually located in the anterior or lateral RA wall. Less common is a MAT circuit revolving around an atrial septal defect closure patch (Figure 7). Although rare, unexcitable areas can sometimes be found, indicating scarring on the RA lateral wall as the MAT substrate in patients without prior surgery (Figure 7).18, 19 It has been suggested that these low-voltage areas may reflect areas of fibrosis, although their nature remains poorly understood.
In its simplest form, scar MAT is a circuit located in the lateral RA wall in whose center there is a line of block consisting of the scar itself (Figure 6, Figure 7, Figure 11). The lower turning point located between the end of the scar and the inferior vena cava is narrow and is often a slow conduction zone. The upper turning point may be the end of the scar or the orifice of the superior vena cava, which can be functionally part of the central obstacle (Figure 6, Figure 7). In widely dilated atria, especially after Fontan surgery, unexcitable low-voltage areas can be found in the RA walls (Figure 11) that contribute to channeling MAT circuits.21, 22, 23 In patients undergoing the Mustard or Senning procedure, in addition to the complexity of the scar barriers, there is the enormous difficulty of accessing the portions of the atria isolated by the patches used to redirect blood flow.24, 25
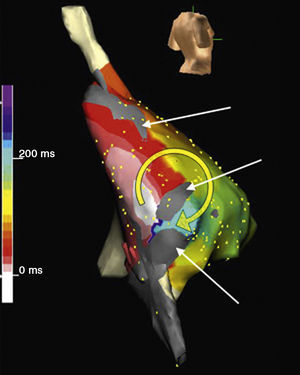
Figure 11. Electroanatomic mapping (Navx® system) of macroreentrant atrial tachycardia in a patient undergoing intervention for tricuspid atresia (right posterolateral view). Note the low-voltage unexcitable areas (gray). The time scale arbitrarily marks the starting point (white) behind an isthmus between two unexcitable areas. Activation revolves around one of the unexcitable areas (white to red→yellow→green→blue) to re-pass the isthmus. Radiofrequency application at this point interrupted the tachycardia.
One of the most important lessons of scar MAT is that it can be avoided if surgical atriotomy is extended to a fixed anatomical obstacle, which would be the inferior vena cava in lateral RA atriotomy. Heart transplantation is a very special case in which the section of the donor's posterior RA wall creates an experimental preparation for the production of typical AF, which explains the fact that this arrhythmia is common in transplanted hearts; however, this could be prevented if the donor CTI underwent ablation (section, cryosurgery, radiofrequency) during the same procedure, taking care to protect the right coronary artery.
Macroreentrant Left Atrial TachycardiaThe anatomical structure of the LA does not contain the major obstacles of the RA, which is the probable reason for MAT being rare in the LA. In patients without prior surgery, macroreentrant left atrial tachycardia (MLAT) occurs in the presence of large unexcitable low-voltage areas and areas of conduction block forming the central nucleus of the circuit, either alone or in connection with an anatomical obstacle (orifices of the pulmonary veins or auricle) (Figure 12, Figure 13).17, 26, 27 The MLAT is associated with organic heart disease (cardiomyopathy, valve disease) and severe patterns of interatrial conduction disorder (Bachmann's bundle block).28 Surgical atriotomy for mitral valve surgery29 or surgical atrial fibrillation ablation30 are common substrates of MLAT. In recent years, the upsurge in atrial fibrillation ablation, involving the destruction of large areas of atrial myocardium, has led to a high incidence of MLAT in these patients,31, 32, 33 leading to the need for new ablation procedures in a large number of them.
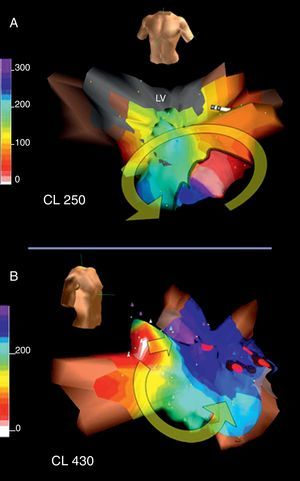
Figure 12. A, Electroanatomical mapping (Navx® system) in the anterior view of a macroreentrant atrial tachycardia circuit revolving around the mitral annulus in patients with no previous surgery; note the wide low-voltage unexcitable area (in gray) in the posterosuperior left atrium. B, electroanatomical mapping in the right anterior oblique view of a very slow focal atrial tachycardia (cycle, 430ms) inducible after interruption of macroreentrant atrial tachycardia with radiofrequency applications between the low-voltage area and the mitral annulus (red dots). This focal atrial tachycardia originates in the right superior pulmonary vein (white area); as in the case shown in Figure 6, a line of block forces the activation wavefront to revolve ending on the other side of area of block (blue-violet). CL: cycle length; LV, low-voltage.
Figure 13. Electroanatomical mapping (Navx® system) in the anterior view (A) and right posterolateral view (B) of macroreentrant atrial tachycardia rotating around the right superior pulmonary vein. Note the low-voltage unexcitable areas, especially in the posterior left atrium.
Clinical diagnosisWhen the ECG shows tachycardia in a patient without heart disease, with paroxysmal presentation and well-defined P waves at rates between 180 bpm and 220 bpm, and it can be observed that atrioventricular (AV) conduction is not constant (Wenckebach periodicity or 2:1 block) during tachycardia, the most likely diagnosis is FAT. If the rate accelerates at the beginning of the tachycardia and decelerates before spontaneous termination (the “warm-up and cool-down” phenomenon), a focal mechanism due to abnormal automaticity is almost certain. If the patient does not show spontaneous AV block, this can be induced by vagal maneuvers or intravenous injection of adenosine triphosphate (ATP) or adenosine. The interruption of the tachycardia using these maneuvers increases the likelihood that the mechanism is a reentry involving the AV node (intranodal or by accessory pathway).
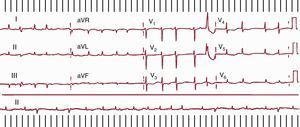
When the ECG records a tachycardia >240 bpm with a pattern typical of counterclockwise or clockwise AF (already mentioned) and fixed or variable AV block in a patient with or without organic heart disease and with no prior cardiac surgery, then the diagnosis of CTI-dependent typical AF is almost certain. If the waveform morphology is not well defined, the degree of AV block can be increased by carotid massage or an injection of ATP or adenosine, while making recordings of the limb leads and lead V1. In patients with prior surgical atriotomy the typical ECG pattern of AF is no longer specific (Figure 14).
Figure 14. Electrocardiogram of a patient with surgical atriotomy. Left, Typical atrial flutter with clockwise rotation in leads II, III and aVF resembling typical atrial flutter with counterclockwise rotation, but with the typical W waves in V1. Right, The electrocardiogram pattern is highly suggestive of typical atrial flutter with counterclockwise rotation, but the macroreentrant atrial tachycardia circuit revolves around the scar on the lateral right atrium without the participation of the cavotricuspid isthmus. AF, atrial flutter; MAT, macroreentrant atrial tachycardia; RA, right atrium.
Given an ECG pattern of atypical AF, the possibilities are many, including FAT or MLAT, especially when there has been prior cardiac surgery or ablation of the atrial myocardium for the treatment of atrial fibrillation. In patients with complex atrial lesions, not even the pattern of typical AF can predict the mechanism of tachycardia; FAT and MAT mechanisms may also be present.
Electrophysiological diagnosis Establishing an Atrial MechanismIf the tachycardia is not present at baseline, it is induced by programmed stimulation with or without infusion of isoproterenol. If the tachycardia does not show spontaneous AV block, its atrial origin is demonstrated by ventricular pacing, which can dissociate the ventricular tachycardia rate without modifying it.
Atrial Activation PatternOnce the atrial mechanism is confirmed, atrial activation mapping is performed to determine the location of the focus of origin of a FAT (Figure 4, Figure 6, Figure 12) or the anatomical configuration of a MAT circuit, locating the key isthmus or isthmuses whose ablation will interrupt it (Figure 6 and Figure 11, Figure 12, Figure 13). The mapping technique is based on measuring the activation delays of multiple points of one or both atria in relation to a time reference that can be the beginning of the P wave or a stable endocardial recording point taking 0 as a reference (arbitrary). Multipolar catheters can obtain several simultaneous recordings as a reference (Figure 15), monitor the stability of the circuit, and show the percentage of the cycle covered by the atrial activation, which is usually <50% in FAT, whereas this can be 100% in MAT if sufficient recordings are obtained (Figure 1, Figure 8, Figure 16, Figure 17). When the foramen ovale is not patent, recordings can be made from the coronary sinus to obtain the activation times near to the inferior mitral annulus and from the right pulmonary artery to record LA roof activation (Figure 15), but mapping the LA in detail requires transseptal catheterization.
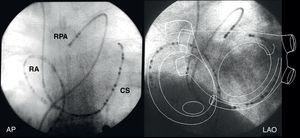
Figure 15. Fluoroscopic images of multipolar electrode catheters in the right atrium, coronary sinus and right pulmonary artery in the anteroposterior and left anterior oblique views. The diagram of the atrial anatomy shows the areas recorded by these catheters. AP, anteroposterior; CS, coronary sinus; LAO, left anterior oblique; RA, right atrium; RPA, right pulmonary artery.
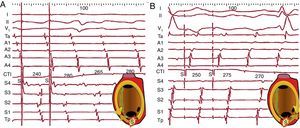
Figure 16. A, Entrainment of typical atrial flutter (counterclockwise) by pacing (S) the cavotricuspid isthmus. Activation of the anterior right atrium is descending (Ta→A4) during the stimulation cycle and during the interruption period, demonstrating that stimulated wavefronts follow the circuit. In each cycle a second wavefront is produced in the opposite direction to the circuit (antidromic) that collides with the front entrained in the previous cycle (scheme). B, The same phenomenon with a reverse sequence in clockwise typical atrial flutter. The septal right atrium shows a decreasing sequence (Tp-S4) during entrainment and after stimulation. The first cycle (return) after stimulation is the same as the spontaneous cycles of atrial flutter in both cases and there is no observable change in morphology on electrocardiogram nor in the sequence in the electrograms (concealed entrainment without fusion), which is typical of a narrow isthmus of a macroreentrant atrial tachycardia circuit. CTI: cavotricuspid isthmus.
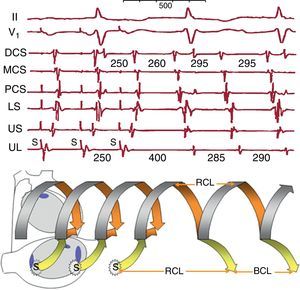
Figure 17. Entrainment of a macroreentrant atrial tachycardia by pacing the upper lateral right atrium. Recordings of the upper septal (US) and lower septal (LS) right atrium and proximal, middle and distal coronary sinuses. Diagram (lower part) representing the time-course of macroreentrant atrial tachycardia circuit in the left atrium in the form of a helix. The pacing wavefront (yellow arrow) reaches the circuit and penetrates it in orthodromic and antidromic directions in each cycle, and by terminating pacing the circuit is restored to its basal cycle. The recordings show the reversal of the upper lateral, upper septal, lower septal and proximal coronary sinus electrogram sequences during pacing (S), whereas the distal coronary sinus→middle coronary sinus sequence is maintained (fusion of the pacing wavefront and entrainment in the circuit). Terminating pacing returns the macroreentrant atrial tachycardia to the baseline cycle of 295ms, as the distal coronary sinus and middle coronary sinus show. However, at the point of stimulation (upper lateral) the return cycle length is longer because, being outside the circuit, the conduction times to and from the circuit are added (yellow arrows). BCL, basal cycle length; DCS, distal coronary sinus; LS, lower septal; MCS, middle coronary sinus; PCS, proximal coronary sinus; RCL, return cycle length; UL, upper lateral; US, upper septal.
A manual point-to-point mapping procedure, annotated over a diagram of the atrial anatomy, is possible in many RA tachycardias, more accessible and with large obstacles that leave few alternatives other than MAT; however, variations in the MAT circuits in the LA are such that they can only be clearly defined by computerized mapping supported by a navigation system to locate the position of the exploring electrodes. Computerized navigation systems translate the time scale into a color scale making the propagation of activation easier to read. Even with these tools, it is not easy to draw these circuits, as they may become unstable when moving catheters for mapping purposes or during programmed stimulation to study entrainment data.
Stimulation During Tachycardia: Transient EntrainmentAtrial pacing at higher frequencies than that of the tachycardia is very useful to confirm a MAT mechanism and to confirm whether a particular point forms part of the circuit. However, since pacing maneuvers can interrupt the tachycardia or alter its mechanism, the ideal approach is to make an initial activation map and confirm the results of this pacing at a minimum number of selected points at rates very close to that of the tachycardia. Recording multiple points from both atria during pacing tests helps to detect the fusion of activation fronts characteristic of MAT, and also to approximately locate the circuit by shifting detailed activation mapping from one atrium to the other.
In FAT, pacing above the rate of the focus completely captures the atria, and by interrupting pacing the FAT can resume after a pause due to suppressing the focal automatism by pacing. If the origin of the FAT can be recorded, the sustained spontaneous activity can sometimes be observed, which is not captured during pacing (entrance block) (Figure 6A). In contrast, in MAT the transient entrainment phenomenon can be observed; that is, during pacing the atria are completely captured, but in such a way that the reentrant activation sequence is sustained, at least in part (Figure 16, Figure 17). The circuit is normally referred to as being entrained, but this phenomenon can also be conceived as the paced wavefronts being entrained in the anatomical structure of the circuit, instead of radially propagating from the point of stimulation. The coexistence of a MAT activation wavefront and another wavefront stimulated in each cycle is known as constant fusion, a phenomenon only possible in MAT (Figure 17). Fusion may not be easily detected when a narrow area of the circuit is paced, such as the CTI in typical AF, which is then referred to as concealed entrainment (no fusion) (Figure 16).
If the circuit does not become unstable or is not interrupted when MAT pacing is interrupted, the CL and baseline activation sequence are restored, and the length of the first return cycle after pacing allows us to determine if the stimulated point is within circuit (return CL=baseline CL) (Figure 16) or some distance from it (return CL > baseline CL) (Figure 17). This maneuver is key to locating the critical isthmus of the circuit and directing radiofrequency applications during ablation.
Drug treatment and ablation Focal Atrial TachycardiaFAT may occur in the absence of heart disease, as demonstrated by noninvasive and invasive examination, and is relatively frequent in young people or children, but is also seen in diseases such as cardiomyopathy, surgically treated congenital heart disease, or in the elderly in the setting of bradycardia-tachycardia syndrome. The underlying pathological mechanism has been little studied. In the few cases derived from surgical or autopsy series, structures similar to a sinus node or inflammation, fibrosis, or perimyocarditis sites have been reported, whereas in other cases no significant abnormalities have been found.34, 35, 36 Recently, much attention has been paid to the anatomic substrate of focal discharges from the pulmonary veins in the setting of atrial fibrillation, and the origin of the focal discharge has been attributed to the myocardial fibers partly isolated by fibrous tissue in the venous sleeves.37, 38, 39 However, although it has long been known that some FATs have their origin in the pulmonary veins, the clinical context is different and there could be different substrates. Detailed studies of the spread of activation in FAT have shown long trajectories from the focus to the point of initiation of radial activation of the atrial myocardium40 along a structure which electrically isolates the stimulus, which could indicate that the foci of the tachycardia may originate in areas of atrial myocardium relatively isolated by fibrosis, inflammation, or a structural abnormality.
Although FAT often manifests as a paroxysmal tachycardia crisis in normal heart and in these cases can be empirically treated with beta blockers, calcium antagonists (diltiazem, verapamil), or antiarrhythmic drugs (amiodarone, flecainide, propafenone, sotalol), the possibility of curative catheter ablation after electrophysiologic study remains very attractive. In very young children (less than 3-4 years of age) it may be worth trying a control drug because FAT can disappear over time.41 It is important to assess FAT that occurs in the setting of dilated cardiomyopathy, because this may be due to the FAT itself (tachycardiomyopathy) and normal cardiac function could be recovered after ablation.42 In patients with complex congenital heart disease treated by surgery, it may be necessary to intervene on several tachycardia mechanisms in one or more procedures. In 2009, a total of 8546 ablation procedures were recorded in Spain; 283 of these were for FAT.43
Catheter ablation of the FAT may be performed using radiofrequency application at the point of earliest endocardial activation. When the focus is close to endocardium, ablation can be achieved using solid 4-mm catheters, but if the focus is epicardial irrigated-tip catheters may be required for better penetration of the lesion. Some cases may require epicardial ablation, either surgical or by pericardial puncture. Before applying radiofrequency, high-intensity stimulation (10-20mA) with the ablation catheter should be performed to ensure that it is not near the phrenic nerve and to prevent damaging it.
The induction of FAT can be difficult and sometimes the pressure of the catheter itself can interrupt it, rendering radiofrequency application impossible during tachycardia. In these cases a computerized mapping and navigation system can identify the location of the focus and guide radiofrequency applications. This difficulty also makes the success of ablation uncertain in some cases and can lead to recurrences after ablation.43 The need for transseptal access for the ablation of FAT originating in the LA can be a limiting factor in some catheterization laboratories.43
Typical FlutterTypical AF preferentially affects men (80%) and may be paroxysmal or persistent. The clinical manifestations strongly depend on AV conduction, which tends to be 2:1 in most cases, with a regular ventricular rate around 140 to 150 bpm, and is often poorly tolerated due to heart palpitations, dizziness, dyspnea, or chest pain. Some patients do not experience symptoms until the sustained high rate causes ventricular systolic dysfunction (tachymyocardiopathy) with end-stage heart failure. The ventricular rate can be very difficult to control in typical AF and controlling clinical symptoms may require electrical cardioversion.
The natural history of typical AF is often interlinked with atrial fibrillation. A follow-up study over 8 to 10 years showed that more than 50% of patients with AF experienced some episode of atrial fibrillation44; on the other hand, it is very common for patients treated with antiarrhythmic drugs for atrial fibrillation to experience episodes of AF.45 In patients who have episodes of AF and atrial fibrillation, the risk of embolism is the same as in patients with atrial fibrillation alone, whereas patient with AF alone have a lower risk.44, 46 Despite this, chronic anticoagulation therapy for AF is recommended, applying the same criteria as atrial fibrillation.47
Class I antiarrhythmic drugs (procainamide, flecainide, propafenone) are rarely effective for the treatment of typical AF.48, 49 They can also act as arrhythmogenics by slowing the AF rate, providing 1:1 AV conduction at rates ≥200 bpm with a widening of the QRS similar to ventricular tachycardia.45, 50 Antiarrhythmic K+ channel blockers, such as ibutilide48, 49 (unavailable in Spain), are very effective for the treatment of AF and are capable of cardioversion to sinus rhythm in 80% of cases after intravenous administration. However, arrhythmogenesis secondary to QT interval prolongation and the possible induction of polymorphic ventricular tachycardia (torsades de pointes) should be monitored. Electrical cardioversion is effective51 and can be performed using direct current or atrial pacing with a temporary catheter or implanted pacemaker.52
The effectiveness and few complications of CTI catheter ablation make it the treatment of choice for typical AF, especially if it is recurrent.53 In 2009, there were 1859 CTI ablation procedures in Spain, with a 95% success rate.43 A CTI ablation is the creation of a line of necrosis through the CTI from the tricuspid annulus to the inferior vena cava (Figure 7).54 It is important that the result of ablation is complete bidirectional CTI conduction block, demonstrated by pacing and recording techniques on both sides of CTI, because it prevents recurrence of AF in 95% of cases, whereas simply inducing prolonged conduction delays or unidirectional block is followed by a 30% to 50% risk of recurrence.55, 56 Complications of the procedure are rare.53, 54, 55, 56, 57, 58, 59
When the ECG pattern is characteristic and there is no prior cardiac surgery, CTI ablation can be performed without further demonstration of the AF mechanism. In cases where the morphology is less clear or associated disease suggests the presence of atypical MAT circuits, whether associated with typical AF or not, electrophysiological study of the clinical tachycardia is required, whether they are spontaneous or induced during the study through baseline stimulation or during isoproterenol infusion. Using a single multipolar electrode catheter introduced from the inferior vena cava, the circular activation sequence of the RA can be demonstrated and the steerable catheter used for radiofrequency application will record activation in the CTI, forming a “bridge” between anterior RA activation and the lower septal RA, whatever the direction of rotation (Figure 16). Although the map thus obtained is very expressive, the involvement of the CTI should be confirmed by its stimulation, ensuring that the local cycle after stimulation is ≤20ms longer than the baseline AF. During CTI entrainment sustained circular activation in the majority of the RA can be tested, showing that the stimuli are entrained in the circuit (Figure 16).
Long-term follow-up after ablation shows a 30% to 40% incidence of atrial fibrillation and is more common in patients with previously documented episodes.57, 58, 59 This indicates that the AF and fibrillation share common pathogenic factors and that the interruption of the CTI may not prevent the development of atrial remodeling common to both arrhythmias, and which is detectable in the form of conduction delays.14, 60 It is clear that the CTI is not the cause of typical AF, but is an essential part of the MAT circuit which makes it susceptible to catheter ablation, such that the ablation does not modify the electrophysiological and anatomical abnormalities that led to the AF onset and that could progress to fibrillation. These considerations lead us to conclude that effective long-term treatment of typical AF, similar to that proposed for atrial fibrillation, should definitely include secondary prevention measures aimed at treatable pathogenic factors (hypertension, bronchopneumopathy, sleep apnea) to maintain stable sinus rhythm in the long-term. Researching effective methods for secondary prevention should be a priority task in the near future.
Macroreentrant Tachycardia/Atypical FlutterIn 2009, the Spanish Catheter Ablation Registry recorded a total of 201 macroreentrant atrial tachycardia/atypical ablation atrial flutter procedures.43 These tachycardias are more difficult to study; the incidence of complications is higher and the results of ablation are less clear than in FAT or typical AF. Given the aim of this study, we deal with them separately according to their anatomical location in the RA or LA.
Macroreentrant Right Atrial TachycardiaNon-CTI-dependent MAT can occur in the RA, especially in patients with prior surgical atriotomy, either for congenital disease (atrial or ventricular septal defect, Fontan surgery, Mustard or Senning procedure) or acquired disease (myxoma, tricuspid valvular disease). MAT circuits form around barriers consisting of surgical scars and are usually located in the lateral or posterolateral RA wall with a turning point almost always located between the lower edge of the scar and the inferior vena cava, forming the critical isthmus of the circuit and the target of ablation (Figure 7).61 Postoperative RA MAT is almost invariably associated with typical AF, such that definitive treatment should include the ablation of the isthmus between the scar and the inferior vena cava of the lateral wall and that of the CTI. In MAT based on low-voltage unexcitable areas in the RA, with no prior surgical history, the circuit can be interrupted by ablation of a critical isthmus, but published reports are scarce and the long-term outcome is uncertain.18, 19
In patients who have undergone Fontan surgery, there may be simple MAT circuits around sutures. However when the RA is very dilated multiple low-voltage unexcitable areas can form with the potential to sustain multiple circuits, in which case ablation therapy is not always completely successful21, 22, 23 and there also may be associated focal tachycardias. Tachycardia recurrence in these patients is very common in the long term.
Macroreentrant Left Atrial TachycardiaElectrophysiological study and the ablation of MLAT entails the risks involved using a transseptal approach and possible systemic embolism during catheter manipulation in the LA.43 It is advisable to rule out the presence of thrombi by transesophageal echocardiography before the procedure. It is a complex procedure that requires a comprehensive system and careful preparation and interpretation of the activation maps obtained. The movement of catheters or stimulation can interrupt or alter the circuit, which sometimes makes it impossible to define. Despite these drawbacks, the tachycardia can be interrupted in many cases, but it is relatively common to induce another MAT or FAT (Figure 12). The long-term prognosis of ablation of MLAT remains uncertain and a high incidence of atrial fibrillation at follow-up in the mid-term has been described.17, 26, 27 Given the risks and the uncertain long-term prognosis, indications for electrophysiological study and ablation of MLAT should be limited to patients who do not respond to medical treatment and who have poor tolerance of tachycardia, and in whom the risk-benefit ratio may be favorable.
Rate control strategiesA strategy to control the ventricular rate is a valid option in many patients who can sufficiently tolerate the tachycardia. Digoxin, beta blockers, and calcium antagonists, alone or in combination, may keep the ventricular rate within acceptable limits based on clinical assessment. In cases where the rate cannot be satisfactorily controlled, AV node ablation and the implantation of a rate-response pacemaker may be indicated.
Multifocal atrial tachycardiaMultifocal atrial tachycardia is a rare arrhythmia, characterized by morphologically variable P waves, irregular intervals, and a rate >100 bpm. Ventricular rate can also become irregular and initially may resemble atrial fibrillation, but closer observation shows P waves separated by isoelectric baselines between intervals (Figure 18).
Figure 18. Multifocal atrial tachycardia in a patient with hypomagnesemia and hypokalemia secondary to postoperative enteric fistula. The rhythm is completely irregular. There are well-defined P waves, although with distinct morphologies, and some are not conducted due to precocity.
Multifocal atrial tachycardia occurs in patients with major homeostasis abnormalities, such as respiratory failure with hypoxemia or severe electrolyte abnormalities (hypokalemia, hypomagnesemia). It also occurs with the administration of phosphodiesterase inhibitors or catecholamines and is attributed to abnormal automaticity due to afterdepolarization.62 In the first months or years of life it may present with symptoms of tachycardiomyopathy and heart failure due to very high frequencies, but may disappear in the medium term.63 The mainstay of treatment is the correction of hypoxemia and electrolyte abnormalities, including the administration of intravenous magnesium sulfate.64 Multifocal tachycardia may respond to beta blockers or verapamil when not contraindicated by hypotension or respiratory failure.
Conflicts of interestDr. García Cosío has received teaching grants from Medtronic, technological support from St. Jude Medical, and funding to attend congresses from St. Jude Medical, Medtronic, and Sanofi. He also acts as a consultant for Bayer and Biotronik.
Received 17 November 2011
Accepted 19 November 2011
Corresponding author: Servicio de Cardiología, Hospital Universitario de Getafe, Ctra. de Toledo Km 12,5, 28905 Getafe, Madrid, Spain. fgarciacosio.hugf@salud.madrid.org