The effect of a centrifugal continuous-flow left ventricular assist device (cfLVAD) on hemodynamic left ventricular unloading (HLVU) and the clinical conditions that interfere with hemodynamic optimization are not well defined.
MethodsWe retrospectively evaluated the likelihood of incomplete HLVU, defined as high pulmonary capillary wedge pressure (hPCWP)> 15mmHg in 104 ambulatory cfLVAD patients when the current standard recommendations for cfLVAD rotor speed setting were applied. We also evaluated the ability of clinical, hemodynamic and echocardiographic variables to predict hPCWP in ambulatory cfLVAD patients.
ResultsTwenty-eight percent of the patients showed hPCWP. The variables associated with a higher risk of hPCWP were age, central venous pressure, absence of treatment with renin-angiotensin-aldosterone system inhibitors, and brain natriuretic peptide levels. Patients with optimal HLVU had a 15.2±14.7% decrease in postoperative indexed left ventricular end-diastolic diameter compared with 8.9±11.8% in the group with hPCWP (P=.041). Independent predictors of hPCWP included brain natriuretic peptide and age. Brain natriuretic peptide <300 pg/mL predicted freedom from hPCWP with a negative predictive value of 86% (P <.0001).
ConclusionsAn optimal HLVU can be achieved in up to 72% of the ambulatory cfLVAD patients when the current standard recommendations for rotor speed setting are applied. Age, central venous pressure and therapy with renin-angiotensin-aldosteron system inhibitors had a substantial effect on achieving this goal. Brain natriuretic peptide levels and the magnitude of reverse left ventricular remodeling seem to be useful noninvasive tools to evaluate HLVU in patients with functioning cfLVAD.
Keywords
Continuous-flow left ventricular assist devices (cfLVAD) have become a standard therapy for patients with advanced chronic heart failure (HF), both as a bridge to heart transplant (BTT) or as destination therapy. cfLVAD support has been demonstrated to improve functional class, organ perfusion and HF symptoms by unloading the left ventricle (LV) and improving hemodynamics.1 Despite these proven benefits, left ventricular assist device (LVAD) support is still associated with adverse events that lead to morbidity, hospital admissions,2 and considerable costs.3 Normalization of hemodynamics seems to play an important role in achieving a clinical benefit after the implantation of a cfLVAD. It has been reported that a decrease in pulmonary capillary wedge pressure (PCWP) as a consequence of left ventricular unloading (LVU) is related to better functional class, lower HF admissions, and better survival free of any hemocompatibility-related adverse events.4,5
The rotor speed setting (Rsp) for LVAD during follow-up is a critical factor that determines the amount of LVU. This speed adjustment is crucial to achieve a compromise between the degree of LVU and the operation of the LVAD within optimal physiological ranges that promote good functional class, without excessive negative pressure in the LV cavity, resulting in ventricular suction that can lead to arrhythmias and right ventricular (RV) dysfunction.
This compromise is generally achieved with noninvasive methods that avoid the potential drawbacks of repeated invasive assessments. Nevertheless, it is still a major challenge to obtain an optimal Rsp because LVU, at a given Rsp, may also vary with the stage of HF and several preload and afterload conditions. Physical examination is the most accessible tool but it has low sensitivity in assessing hemodynamics compared with right heart catheterization (RHC).6 In this scenario, clinical guidelines recommend transthoracic echocardiography (TTE) as an integral part of determining optimal Rsp.7 That the serial measurement of LV end-diastolic diameter (LVEDD) with TTE, combined with the degree of aortic valve (AV) opening, can be used as a surrogate marker for LVU seems intuitive and is supported by limited literature, derived primarily from HeartMate II studies. However robust outcome data are limited and their applicability to centrifugal cfLVAD patients has not been demonstrated at this time. Moreover, the effects of other factors that have proved their association with LVU in scenarios outside the LVAD field, ie, age or renal function, have not been studied in this clinical setting.
The aim of this study was to evaluate the likelihood of an effective hemodynamic LVU (HLVU) in ambulatory patients with a functioning centrifugal cfLVAD implanted as BTT when the current standard recommendations for Rsp optimization were applied. The association of an incomplete HLVU with different clinical, biochemical and echocardiographic conditions and its impact on mortality was also studied.
METHODSStudy designWe performed a retrospective study that included consecutive patients who had received a centrifugal cfLVAD (HVAD Heartware [Medtronic, United States] or HeartMate 3 [Abbott, United States]) between January 2016 and June 2019 as BTT and underwent an RHC at least 3 months after LVAD implantation as part of the screening process for heart transplant (HT) candidacy.
The following were considered exclusion criteria: age <18 years, patients who did not undergo an RHC examination or who underwent this examination before 3 months after LVAD implantation, and assist devices other than primary centrifugal cfLVAD. We excluded LVAD as destination therapy because these patients did not routinely undergo an RHC during follow-up at our institution.
Patients were followed up in our outpatient LVAD clinic every 3 months or at any time if they developed LVAD-related events or clinical decompensation. Routine ambulatory follow-up consisted of clinical, electrocardiographic, TTE and hematological examination and included documentation of medication and LVAD settings, readouts, and alarms. We did not routinely perform ramp study in ambulatory stable patients.
Rsp optimization during follow-up was based exclusively on the clinical, echocardiographic and LVAD settings examination. Rsp was increased if clinical signs of congestion and echocardiographic signs of insufficient LVU were present. Rsp was decreased if clinical and echocardiographic signs of isolated RV dysfunction were present and in cases of low-flow alarms or suction events due to volume depletion or RV dysfunction. Rsp was optimized to the minimum speed required to guarantee the best possible clinical status and LVAD flow in the absence of suction events, while maintaining mean arterial pressure between 65 and 85mmHg and echocardiographic signs of LVU, that is, middle interventricular septum (IVS) position and intermittent AV opening with no significant mitral regurgitation.
Treatment with a specific HF medication consisting of neurohormonal blocking agents, that is, concurrent beta-blocker, mineralcorticoid-receptor antagonist and renin-angiotensin-antagonist system inhibitor (RAASi) in the form of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker was used up to the maximum tolerated dose in all patients unless clinical or hemodynamic intolerance was present. Diuretics were optimized to the lowest possible dose according to volume status and renal function. All patients with preoperative severe tricuspid regurgitation underwent tricuspid valve repair (TVR) at the time of cfLVAD implant.
All data were retrospectively collected from clinical records and analyzed. The surveillance time finished at the end of the study follow-up (end of September 2020) or if HT or death occurred. The study was approved by the institutional ethics committee (ID number of the IRB approval: 2020-622) on 11th August 2020. There was no requirement for informed patient consent.
VariablesAll patients underwent an RHC in a stable clinical condition. The hemodynamic values obtained preoperatively and after cfLVAD implantation are described in table 1 and table 2, respectively. We did not perform repeat RHC to re-evaluate hemodynamics.
Preoperative clinical, hemodynamic and echocardiographic characteristics stratified by hemodynamic left ventricular unloading during left ventricular assist device support
| Global (104 patients) | PCWP ≤ 15 (75 patients) | PCWP> 15 (29 patients) | P | |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age, y | 55 [49-61] | 54 [46-60] | 57 [53-61] | .038 |
| Male sex | 91 (87.5) | 65 (86.6) | 26 (89.6) | .685 |
| Female sex | 13 (12.5) | 10 (13.3) | 3 (10.3) | |
| BMI | 26 [22-29] | 25 [22-28] | 27 [22-30] | .223 |
| Dilated cardiomyopathy | 64 (61.5) | 48 (64) | 16 (55.1) | .523 |
| Ischemic cardiomyopathy | 40 (38.4) | 27 (36) | 13 (44.8) | |
| INTERMACS 1 or 2 | 63 (60.6) | 50 (66.6) | 13 (44.8) | .051 |
| Creatinine, mg/dL | 1.3 [0.9-1.8] | 1.1 [0.9-1.7] | 1.5 [1.1-2.2] | .040 |
| Hemodynamic characteristics | ||||
| CVP, mmHg | 13 [10-18] | 13 [9.3-18] | 12.5 [10-18.5] | .738 |
| mPAP, mmHg | 35.5±10.5 | 34.9±11 | 36.8±10 | .455 |
| CI, L/min/m2 | 1.8 [1.3-2.2] | 1.6 [1.1-1.9] | 1.9 [1.5-2.4] | .283 |
| PVR, dyn/seg*cm5 | 215 [157-318] | 229 [92-324] | 202 [151-314] | .468 |
| dTPG, mmHg | 2 [1-4] | 2 [1-4] | 1.5 [1-4.7] | .284 |
| PCWP, mmHg | 24.5±8.3 | 24.8±8.7 | 24.1±7.9 | .755 |
| CVP/PCWP | 0.55±0.23 | 0.53±0.22 | 0.59±0.24 | .316 |
| PPi: (sPAP-dPAP)/CVP | 1.9 [1.3-3] | 1.6 [1.2-3] | 2.3 [1.5-3] | .384 |
| RVSWI, mmHg*mL/m2 | 445 [285-630] | 373 [238-554] | 588 [360-804] | .010 |
| Echocardiographic characteristics | ||||
| LVEDD, mm | 70±12 | 69.6±11.6 | 70±12.3 | .888 |
| Indexed LVEDD, mm/m2 | 33 [30-37] | 32.8 [29.9-38.9] | 33.9 [31-35] | .94 |
| EDDRV1, mm | 42±10 | 41.4±10 | 43.8±9.5 | .283 |
| TAPSE, mm | 15.8±4.7 | 15.4±5 | 16.7±3.4 | .243 |
| Severe TR | 14 (13.4) | 7 (9.3) | 7 (24.1) | .047 |
BMI, body mass index; CI, cardiac index; CVP, central venous pressure; EDDRV1, baseline end-diastolic right ventricular linear dimension in 4 chamber view; LAVD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; mPAP, mean pulmonary pressure; PCWP, pulmonary capillary wedge pressure; PPi, pulmonary artery pulsatility index; PVR, pulmonary vascular resistance; RVSWI, right ventricle stroke working index=(PAPm-CVP)*(CI/HR)*1000; sPAP and dPAP, systolic and diastolic pulmonary pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
The data are expressed as No. (%), mean ± standard deviation, or median [quartile 25%-quartile 75%].
Clinical characteristics, hemodynamics, echocardiographic variables and complications after left ventricular assist device implantation stratified by hemodynamic left ventricular unloading
| Overall (104 patients) | PCWP ≤15(75 patients) | PCWP> 15 (29 patients) | P | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| TVR | 14 (13.4) | 7 (9.3) | 7 (24.1) | .047 |
| Atrial fibrillation | 29 (27.9) | 18 (24) | 11 (37.9) | .155 |
| Creatinine, mg/dL | 1.3 [1-1.7] | 1.2 [0.9-1.6] | 1.4 [1.2-2.3] | .017 |
| Hemoglobin, g/dL | 12 [10.5-13.5] | 12.5 [10.6-14] | 11.7 [9.9-12.7] | .066 |
| BNP, pg/mL | 225 [120-562] | 195 [103-322] | 570 [312-986] | <.001 |
| Torasemide, mg/d | 10 [0-20] | 10 [0-20] | 20 [0-35] | .024 |
| Beta-blocker | 99 (95.2) | 73 (97.3) | 26 (89.7) | .101 |
| RAAS inhibitors | 69 (66.3) | 55 (73.3) | 14 (48.2) | .015 |
| MRA | 71 (68.2) | 49 (65.3) | 22 (75.9) | .301 |
| LVAD parameters at the time of RHC | ||||
| LVAD HW | 44 (42.3) | 31 (41.3) | 13 (44.8) | .476 |
| LVAD HM3 | 60 (57.7) | 44 (58.7) | 16 (55.2) | |
| RPM HW* x100 | 27.5 [26.5-29.5] | 27 [26-29] | 27.5 [26.5-29.5] | .803 |
| RPM HM 3* x 100 | 55 [54-57] | 54 [53-56] | 55 [54-57] | .775 |
| Hemodynamics | ||||
| CVP, mmHg | 9 [4-12] | 7 [3-10] | 14 [9-19.5] | <.001 |
| mPAP, mmHg | 19 [15-25] | 17 [14-21] | 28 [24-37] | <.001 |
| CI, L/min/m2 | 2.3 [2-2.5] | 2.3 [2-2.5] | 2.3 [2-2.5] | .813 |
| Ven O2 sat | 65.7±7 | 65.9±7 | 65.1±8 | .667 |
| PVR (dyn/seg*cm5 | 122 [92-182] | 121 [92-180] | 123 [79-214] | .945 |
| PCWP, mmHg | 12 [8-16] | 10 [7-12] | 21 [17-26] | <.001 |
| CVP/PCWP | 0.75 [0.45-1] | 0.8 [0.5-1.1] | 0.6 [0.5-0.9] | .101 |
| HR, bpm | 74±14 | 73±14 | 76±14 | .755 |
| Mean blood pressure, mmHg | 75±9 | 77±8 | 72±10 | .137 |
| PPi: (sPAP-dPAP)/CVP | 2 [1.4-3.4] | 2.1 [1.5-4.2] | 1.6 [1-2.6] | .017 |
| RVSWI, mmHg*mL/m2 | 359±198 | 316±152 | 468±255 | .005 |
| Echocardiographic characteristics at the time of RHC | ||||
| Indexed LVEDD, mm/m2 | 29.1±5.3 | 28.6±5.4 | 30.3±4.9 | .149 |
| Presence of indexed LVEDD dilatation, % | 28 (26.9) | 18 (24) | 10 (34.5) | .280 |
| Delta-indexed LVEDD change, mm/m2 | 4.1 [2-7.5] | 5 [2.4-9] | 2.6 [0-6] | .021 |
| Delta-indexed LVEDD change | 13.3 + −14 | 15.2 + −14.7 | 8.9 + −11.8 | .043 |
| EF LV <30 | 92 (88.4) | 65 (86.6) | 27 (93.1) | .357 |
| AV opening | 38 (36.5) | 27 (36) | 11 (37.9) | .854 |
| Moderate-severe MR | 6 (5.7) | 4 (5.3) | 2 (6.9) | .759 |
| Moderate-severe AR | 3 (2.8) | 2 (2.6) | 1 (3.4) | .806 |
| EDDRV1, mm | 45 [35-49] | 38 [35-44] | 41 [38-45] | .075 |
| TAPSE, mm | 13.8±3.5 | 13.9±3.4 | 13.7±3.8 | .243 |
| Moderate-severe TR | 13 (12.5) | 8 (10.7) | 5 (17.2) | .363 |
| Outcomes during follow-up | ||||
| Heart transplant | 21 (20.2) | 15 (20) | 6 (20.7) | .937 |
| Stroke | 9 (8) | 7 (9.3) | 2 (6.9) | .679 |
| GI bleeding | 11 (8.6) | 7 (9.3) | 4 (13.8) | .017 |
| Dialysis | 4 (3.8) | 0 | 4 (13.8) | .004 |
| Systemic hypertension | 12 (11.5) | 8 (10.7) | 4 (13.8) | .751 |
| Driveline or device infection | 29 (27.9) | 21 (28) | 8 (27.6) | .820 |
| LVAD thrombosis | 6 (5.7) | 5 (6.7) | 1 (3.4) | .667 |
| HF hospitalization | 12 (11.5) | 7 (9.3) | 5 (17.2) | .268 |
AR, aortic regurgitation; AV, aortic valve; BNP, brain natriuretic peptide; CI, cardiac index; CVP, central venous pressure; EDDRV1, baseline end-diastolic right ventricular linear dimension in 4-chamber view; GI, gastrointestinal; HF, heart failure; HM, heart mate; HR, heart rate; HW, heart ware; LV, left ventricle; LVAD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; mPAP, mean pulmonary pressure; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonists; PCWP, pulmonary capillary wedge pressure; PPi, pulmonary artery pulsatility index; PVR, pulmonary vascular resistance; RAAS inhibitors, renin-angiotensin-aldosterone system inhibitors (angiotensin converting enzyme inhibitors or angiotensin receptor blockers [ACEis/ARBs]); RHC, right heart catheterization; RPM, rate per minute; RVSWI, right ventricle stroke working index=(PAPm-CVP)*(CI/HR)*1000; sPAP and dPAP, systolic and diastolic pulmonary pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; TVR, tricuspid valve repair; Ven O2 sat, venous oxygen saturation.
The data are expressed as No. (%), mean±standard deviation, or median [quartile 25%-quartile 75%].
TTE variables described in table 2 were assessed with an EPIQ 7G (Philips Healthcare, United States) according to clinical guidelines.8 We used linear LVEDD from the 2D parasternal long-axis image as our reference value to assess LV size, as in the guidelines it is considered the most reproducible measure of LV size under LVAD support.7 LVEDD was normalized to body surface area (indexed LVEDD). LV dilatation was defined by a long-parasternal indexed EDDLV> 31mm/m2 in men and> 32mm/m2 in women.8 The frequency of AV opening was assessed by recording multiple cardiac cycles with M-mode with the additional use of color M-mode in the parasternal long-axis view. AV opening was defined if opening was present either with every cardiac cycle or intermittently.
TTE, LVAD settings, electrocardiogram, medication and hematology data refer to the day of the RHC, before the performance of any further LVAD optimization. Incomplete HLVU was defined as a high pulmonary capillary wedge pressure (hPCWP)> 15mmHg. During the remainder of surveillance time, we also documented cause of death, HT, complications related to the LVAD, and hospital admissions due to HF decompensation.
Statistical analysisA retrospective analysis of the prospectively collected data was performed using SPSS version 17.0 (SPSS Inc, United States). Comparisons were performed using either the Student t or the Mann-Whitney U test as appropriate. Normal distribution was analyzed with the Kolmogorov-Smirnov test. Categorical data were compared with the Fisher exact test for 2 x 2 tables, and Pearson chi-square test otherwise. Unless otherwise specified, all data are expressed as mean±standard deviation. All significance tests were 2-tailed, and P <.05 was considered to be statistically significant.
The variables were tested with a univariate logistic regression analysis (LRA) concerning their ability to predict hPCWP. Considering the limited number of patients in the hPCWP group and to avoid issues regarding multiple hypothesis testing, we performed a stepwise backward multivariable LRA that included a preselected number of variables with clinical relevance that were significant in the univariate LRA.
Receiver operating characteristic curves were used to evaluate the discriminatory value of brain natriuretic peptide (BNP) concerning its prognostic accuracy to predict PCWP. The best cutoff value was calculated according to the Youden index = sensitivity (1-specificity).
Kaplan-Meier curves were created to evaluate survival stratified by PCWP, which was defined as continued LVAD support at the end of the study follow-up, with observations censored if the patient underwent HT. Statistical significance between the curves was analyzed using the log-rank test. A stepwise multivariable Cox proportional hazards regression model was used to determine the independent effect of a limited number of risk factors on the hazard of death.
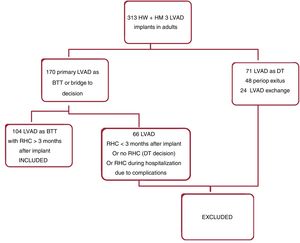
RESULTSA total of 104 consecutive patients were included in the analysis (figure 1). The patients were followed up during a median time of 23±10 months from the time of LVAD implantation. No patients were lost to follow-up.
Baseline preoperative characteristics are described in table 1. Postoperative data are summarized in table 2. None of the patients were treated with sacubitril-valsartan or specific pulmonary vasodilators.
RHC was performed after a median of 10 months (Q1:5, Q3: 15) since LVAD implantation. Seventy-five patients (72%) showed a normal pulmonary capillary wedge pressure (nPCWP) (PCWP ≤ 15mmHg) and 29 patients (28%) had hPCWP (PCWP> 15mmHg).
Patients in the hPCWP group were older, had worse preoperative and postoperative renal function and were more likely to require dialysis after LVAD implantation compared with the nPCWP group. The number of patients treated with RAASi was lower in the group with hPCWP. Requirement for oral diuretics and BNP levels at the time of RHC were significantly higher in patients with hPCWP than in patients with nPCWP. Patients with hPCWP were more likely to undergo concomitant TVR at the time of LVAD implantation due to severe tricuspid regurgitation (table 2).
Postoperative RHC showed higher mean pulmonary pressure and central venous pressure and lower pulmonary artery pulsatility index in patients with hPCWP than in the nPCWP group, but pulmonary vascular resistance showed no significant differences between groups.
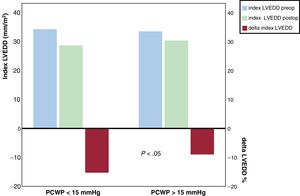
Neither the preoperative nor the postoperative indexed LVEDD showed significant differences between groups. Persistent dilatation of the LV under LVAD support was not statistically associated with the presence of hPCWP. The postoperative indexed LVEDD was strongly correlated with the preoperative indexed LVEDD (R, 0.75; P <.0001). However, the magnitude of the decrease of the indexed LVEDD was more pronounced in patients with nPCWP. The indexed LVEDD decreased 15.2 + −14.7% in the nPCWP group compared with 8.9 + −11.8% in the hPCWP group (P=.043), (figure 2).
Differences in the preoperative and postoperative indexed left ventricular end-diastolic diameter and in the percentage of the reduction of left ventricular end-diastolic diameter after cfLVAD stratified by hemodynamic left ventricular unloading. Comparison between groups for delta-indexed LVEDD showed P <.05. LVEDD preop, preoperative left ventricular end-diastolic diameter; postop, postoperative; delta-indexed LVEDD, percentage of the reduction of left ventricular end-diastolic diameter; PCWP, pulmonary capillary wedge pressure.
We observed a marked decrease in the BNP values after LVAD implantation (median delta 1362, Q1 330, Q3 2651 pg/mL) with higher BNP values at the time of RHC in the hPCWP group.
Regarding LVAD-related complications, patients with hPCWP showed more major gastrointestinal bleeding (GIb) events than patients with nPCWP (table 2).
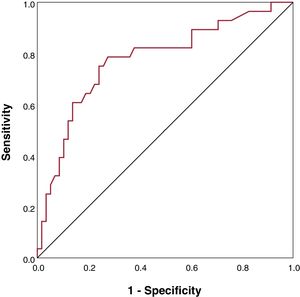
Age, higher central venous pressure and BNP levels were associated with higher risk of hPCWP in the univariate LRA. A higher delta-indexed LVEDD change and treatment with RAASi decreased the odds of hPCWP (table 3). LRA demonstrated that age and BNP were independent predictors of hPCWP (table 3). BNP showed good predictive discrimination in the receiver operating characteristic analysis (figure 3). BNP <300 pg/mL was associated with a reduced risk of hPCWP (odds ratio, 0.14; 95%CI, 0.05-0.4); P <.001) and could anticipate freedom from hPCWP with a predictive value of 86%, 75% specificity, and 75% sensitivity.
Unadjusted univariable and multivariable regression analysis for outcome of hPCWP after LVAD implantation
| Variables | Univariable | Variables* | Multivariable | ||
|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | ||
| Age | 1.07 (1.1-1.2) | .016 | Age | 1.06 (1.01-1.12) | .048 |
| Creatinine preoperative | 1.4 (0.8-2.2) | .144 | |||
| TVR | 3.1 (0.97-9.78) | .060 | |||
| CVP postoperative | 1.2 (1.1-1.3) | .0001 | |||
| mPAP postoperative | 1.5 (1.3-1.8) | .0001 | |||
| Creatinine postoperative | 1.4 (0.9-2.1) | .142 | |||
| Hemoglobin postoperative | 0.8 (0.6-1) | .055 | |||
| PPi postoperative | 0.97 (0.8-1.07) | .619 | |||
| BNP postoperative | 1.1 (1.05-1.2) | .001 | BNP postoperative<300 pg/mL | 0.14 (0.05-0.4) | .0001 |
| Delta-indexed LVEDD change mm/m2 | 0.89 (1.5-20) | .032 | |||
| Indexed LVEDD postoperative | 1.06 (0.9-1.2) | .152 | |||
| RAAS inhibitors | 0.34 (0.14-0.82) | .017 | RAAS inhibitors | -- | .366 |
95%CI, 95% confidence interval; BNP, brain natriuretic peptide; CVP, central venous pressure; hPCWP: high pulmonary capillary wedge pressure; LVAD, left ventricular assist device; LVEDD, left ventricular end-diastolic diameter; mPAP, mean pulmonary pressure; OR, odds ratio; PPi, pulmonary artery pulsatility; RAAS inhibitors, renin-angiotensin-aldosterone system inhibitors (angiotensin converting enzyme inhibitors or angiotensin receptor blockers); TVR, tricuspid valve repair.
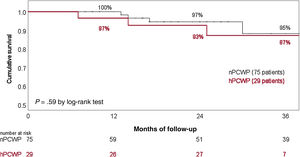
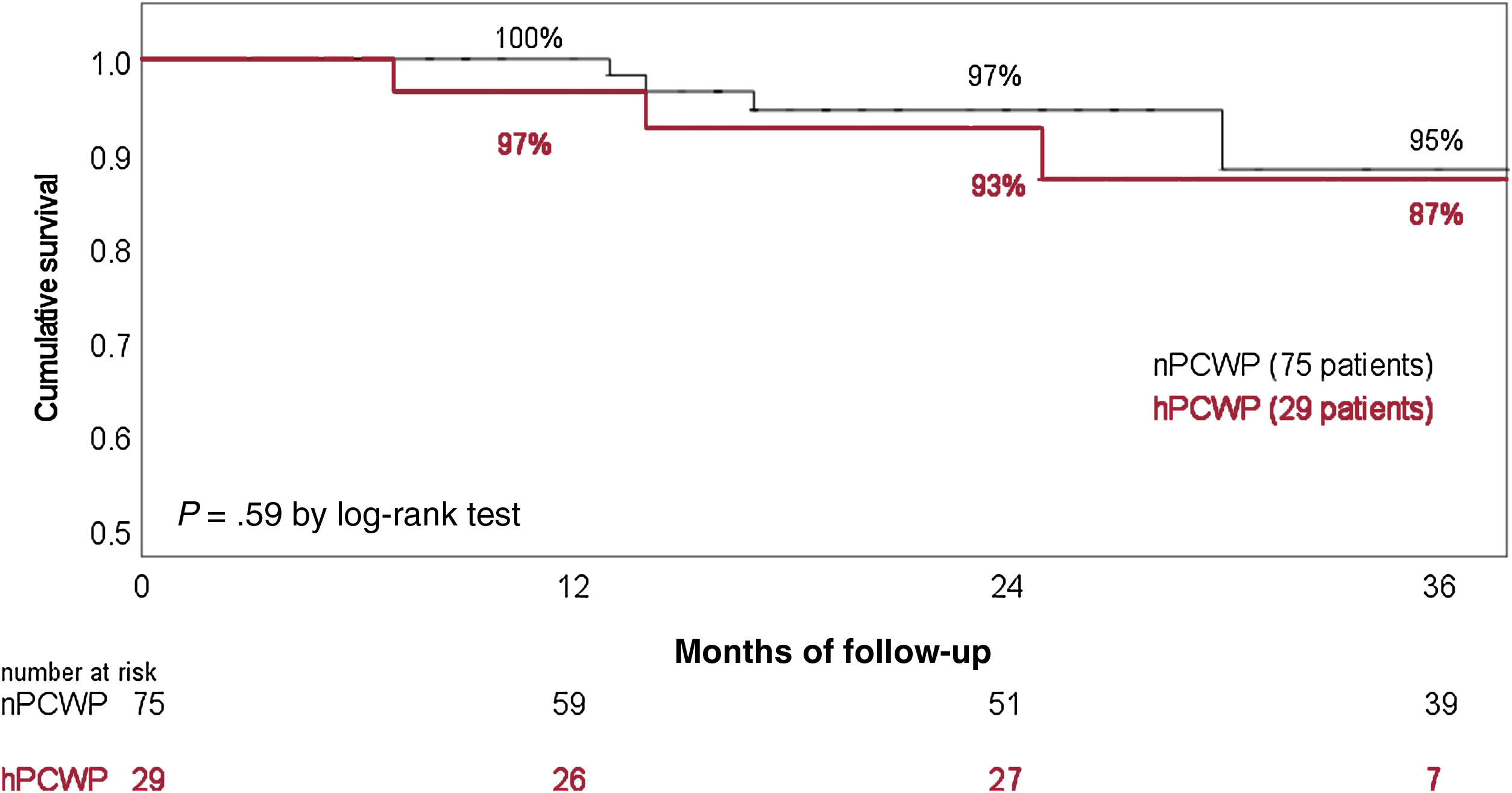
Twelve patients required hospitalization due to HF decompensation during the surveillance time (7 patients from the nPCWP group). Seven patients died during follow-up (4 patients from the hPCWP group, table 4). Twenty-one patients underwent HT, with comparable HT likelihood between groups. Overall survival (41.5 months in nPCWP group vs 39.6 months in the hPCWP group) and survival free from HF hospitalizations (37.5 months vs 36 months respectively, log-rank P=.75) showed no significant differences between groups (figure 4). Cox regression analysis did not show a significant effect from PCWP group, age or treatment with RAASi on survival.
Causes of death and heart failure hospitalizations stratified by hemodynamic left ventricular unloading
| case | PCWP> 15 mmHg | HF hospitalization | Cause of death |
|---|---|---|---|
| 1 | No | Ischemic stroke | |
| 2 | Yes | Acute hepatitis | |
| 3 | No | Infection | |
| 4 | Yes | LVAD thrombosis | - |
| 5 | Yes | Acute renal failure | - |
| 6 | Yes | Acute renal failure | - |
| 7 | No | Unclear | - |
| 8 | No | RV failure | - |
| 9 | No | RV failure | - |
| 10 | No | Systemic hypertension | - |
| 11 | No | Ventricular arrythmia | - |
| 12 | No | TIA | - |
| 13 | Yes | Acute renal failure | - |
| 14 | No | Unclear | - |
| 15 | Yes | Infection | |
| 16 | No | Infection | |
| 17 | Yes | GIb | - |
| 18 | Yes | Tumor | |
| 19 | Yes | Intracranial hemorrhage |
GIb, gastrointestinal bleeding; HF, heart failure; LVAD, left ventricular assist device; PCWP, pulmonary capillary wedge pressure; RV, right ventricular; TIA, transient ischemic attack.
Survival rate stratified by the existence of incomplete hemodynamic left ventricular unloading during cfLVAD support. cfLVAD, continuous-flow left ventricular assist device; hPCWP, high pulmonary capillary wedge pressure> 15mmHg; nPCWP, normal pulmonary capillary wedge pressure ≤ 15mmHg.
Despite the decrease in volume and pressure LV load that follows the implantation of a cfLVAD, the effect of the centrifugal cfLVAD on the HLVU in clinically stable patients has not been fully investigated. Moreover, it is not clear if an insufficient HLVU correlates with clinical HF worsening. To the best of our knowledge, this is the largest clinical study that evaluates HLVU in ambulatory patients supported with centrifugal cfLVAD. This study allows us to conclude that an optimal HLVU can be achieved in up to 72% of the ambulatory patients supported with current LVAD models as BTT when the Rsp is based on current clinical and echocardiographic recommendations. In comparison, a previous study reported9 that a normal HLVU was observed in 43% of clinically stable patients supported with the previous HeartMate II or with Heartware LVAD. In a study of a small cohort of 16 patients with functioning HeartMate 3, Uriel et al.10 reported that 62.5% of the patients had a normal hemodynamic profile. Although the patients from these studies might be not fully comparable, it seems that an optimal HLVU is more frequently achieved in clinical practice with the centrifugal cfLVAD than with older generations of LVAD.
We observed that suboptimal HLVU was significantly associated with fluid overload as reflected in a higher need for diuretic therapy and higher BNP levels. A better understanding of the clinical conditions associated with an insufficient HLVU could help to improve diagnostic protocols during long-term follow-up, which may facilitate an earlier diagnosis and device optimization in selected patients. We observed that the presence of an elevated LV filling pressure was more frequent in older patients with reduced renal function. Moreover, the presence of hPCWP coexisted with elevated central venous pressure, pulmonary pressure, postcapillary pulmonary hypertension and lower artery pulsatility index, which supports the evidence of the strong interaction between the left and right filling pressures that occurs during long-term LVAD support and that can affect long-term RV performance.11 This strong interconnection between filling pressures, age and renal function might perpetuate a pre-existing cardio renal syndrome, despite cfLVAD support, which further aggravates the volume overload and compromises transrenal perfusion pressure and RV function; these factors, which constitute a vicious circle, might promote a maladaptive neurohormonal activation and further renal dysfunction.
Severe preoperative tricuspid regurgitation was more frequent in the hPCWP group, despite the use of TVR at the time of LVAD implantation. The benefit of TVR in protecting the vulnerable RV after LVAD implantation is controversial. Some investigators12 reported that, although preoperative moderate and severe tricuspid regurgitation were associated with poorer survival, TVR did not improve overall survival. Severe tricuspid regurgitation might simply reflect the consequences of long-lasting RV failure and dilatation in patients with HF, which might persist despite TVR. Nevertheless, it may be beneficial, as the baseline characteristics of patients undergoing TVR were suggestive of a more progressive underlying disease.13
RAASi therapy has been extensively demonstrated to produce an effective decrease in the PCWP in patients with chronic HF, and emerging data suggest that guideline-directed medical therapies for HF may also improve clinical outcomes in LVAD patients. We also observed that RAASi were associated with a decreased probability of hPCWP, which further supports its beneficial effect on hemodynamics in LVAD patients. However, while cardiorenal protective, this medication can lead to temporary worsening of renal function in patients with renal dysfunction. In this regard, it is also known that age and reduced renal function significantly increase the risk of clinical intolerance to RAASi in patients with HF, which plausibly occurred to the hPCWP group under LVAD support in our study.14,15
Although RHC during long-term LVAD support would be the reference method for evaluating LVU, clinical guidelines16 currently recommend it only in specific scenarios. In this regard, noninvasive methods for the reliable estimation of hemodynamics would allow for optimization of medical therapy and Rsp without the potential drawbacks of repeated invasive assessments. One of the most attractive candidates for this clinical role would be BNP, which is released from ventricular myocardium as a response to volume expansion or pressure overload and is a strong predictor of mortality and cardiovascular outcomes in HF patients.17,18 A recent study has demonstrated that N-terminal pro-B-type natriuretic peptide and other biomarkers substantially decrease after LVAD implantation but do not regress to normal values.19 However, the authors of that study did not correlate their results with TTE or hemodynamics. In the present study, we demonstrate that BNP levels are persistently elevated above the reference serum levels after LVAD implantation. Furthermore, we provide new evidence that BNP values are independently associated with HLVU. A BNP cutoff point <300 pg/mL was a reliable screening index to rule out hPCWP in ambulatory LVAD patients. Whether this parameter could be broadly used as an additional noninvasive tool to optimize HLVU, limiting the use of invasive RHC, should be investigated in larger and multicenter studies.
The second potential noninvasive clinical tool to estimate hemodynamics is TTE. In this respect, clinical guidelines recommend TTE as an integral component for determining optimal Rsp, with goals including adequate LVU with midline IVS and minimal mitral regurgitation and AV opening.7 However, recent reviews of the management of patients with cfLVAD describe the difficulty of evaluating the degree of LVU with TTE.20 Although some authors have described TTE algorithms to estimate left-side filling pressures,21 optimal results require an expert technique and heavily relies on the E/e′ ratio, which is poorly validated among patients supported with LVAD.22
In clinical practice, serial LVEDD TTE measurements (combined with AV opening) are frequently used as a surrogate marker for the degree of LVU.7 However this assumption is not fully supported by clinical studies. This issue has strong clinical relevance because an unnecessary increase in LVAD speed can have a deleterious effect on volume status and RV function and can trigger arrhythmias.
We observed that the magnitude of the mechanical unloading, which is the percentage of the reduction of the LV dimensions, which translates the degree of LV reversal remodeling, was the only TTE parameter that was significantly associated with the degree of HLVU. Patients with optimal HLVU showed double the magnitude of LV mechanical decompression than the group with persistent hPCWP. Reverse myocardial remodeling is central to the benefits of most HF treatments and is associated with an improved prognosis. Some authors have also demonstrated that reduced concentrations of N-terminal pro-B-type natriuretic peptide after guideline-directed medical therapy are significantly associated with reverse remodeling.23 Our study provides evidence that optimal HLVU after LVAD implantation translates into lower BNP concentrations and corresponding more favorable effects on reverse myocardial remodeling.
In our study, the postoperative indexed LVEDD was not associated with PCWP and showed a strong correlation with the preoperative indexed LVEDD. From a practical point of view, persistent dilatation of the LV may simply reflect the degree of adverse LV remodeling and dilatation prior to LVAD implantation. The presence of mitral regurgitation or the absence of AV opening do not seem to help either in this decision-making in clinically stable patients. The AV opening might reflect the degree of intrinsic improvement in LV function, for instance in patients with LV recovery. Changes in LVAD settings or in HF medication guided by surrogate markers of inefficient HLVU, such as persistent dilatation of the LV, AV opening or mitral regurgitation, seem not to be supported by our findings and might have a deleterious effect on hemodynamics. However, the magnitude of the reverse LV remodeling seems to be associated with the degree of hemodynamic LV decompression. The discriminatory value of these parameters should not be extrapolated to cases of suspected LVAD thrombosis or device malfunction because we only evaluated the diagnostic reliability of these parameters in ambulatory patients with functioning LVAD.
Patients with hPCWP showed more major GIb events. Although the investigation of risk factors for GIb was beyond the scope of our investigation, the greater number of GIb events could be explained by the higher age and central venous pressure, worse renal function and RV performance, and lower RAASi treatment in the hPCWP group.24 However whether an incomplete HLVU could enhance the risk of GIb should be evaluated in future studies.
LimitationsThis study has the following limitations. a) The study power was limited due to the intrinsic nature of a single-center retrospective study. b) We could not demonstrate that HLVU had an impact on HF hospitalizations or survival. However, we did not systematically repeat RHC after further optimizations of the Rsp during follow-up. c) We did not use the results of the RHC to optimize Rsp or HF medication if patients were otherwise clinically stable and showed optimal TTE and LVAD technical parameters. Therefore, our study cannot answer the question of whether changes in HF medication or Rsp based on RHC might impact on hemodynamics. d) We included ambulatory patients with functioning cfLVAD as BTT so the extrapolation of results to other groups is unknown. e) We did not analyze either the duration of AV opening7 or new TTE algorithms to estimate LV filling pressure.22f) Sacubitril-valsartan improves LV remodeling in HF and its benefit on blood pressure control has been evaluated in small series of LVAD patients with trends toward reduced LVEDD and N-terminal pro-B-type natriuretic peptide.25 However its impact on HLVU among patients with LVAD is unknown and there is no prospective evidence of its efficacy or tolerability.
CONCLUSIONSAn optimal HLVU can be achieved in up to 72% of ambulatory patients supported with cfLVAD when current standard recommendations for Rsp are applied. Our study highlights the effect of age, central venous pressure and therapy with RAASi on achieving this goal. BNP levels and the magnitude of the reverse LV remodeling seem to be useful noninvasive tools to evaluate LVU during follow-up in ambulatory patients with functioning cfLVAD support.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSM.J. Ruiz-Cano: Conceptualization, data curation, formal analysis, methodology, validation, visualization, writing—original draft, writing—review and editing. R. Schramm: Conceptualization, validation, visualization. L. Paluszkiewicz: Validation, visualization, data curation, writing—review and editing. L. Ramazyan: Data curation, writing—review and editing. V. Lauenroth: Resources, validation, visualization. S.V. Rojas: Validation, visualization. J. Gummert: Conceptualization, validation, visualization. M. Morshuis: Conceptualization, methodology, supervision, validation, visualization.
CONFLICTS OF INTERESTThe authors have no conflicts of interest to declare regarding this manuscript.
- -
Normalization of hemodynamics after cfLVAD improves functional class and survival free of any hemocompatibility-related adverse events.
- -
Rsp is a critical factor that determines the amount of HLVU. However HLVU may also vary with several preload and afterload conditions.
- -
Currently, there are no reports demonstrating the applicability of some noninvasive methods for Rsp optimization that are usually used as a surrogate marker for HLVU.
- -
An optimal HLVU can be achieved in up to 72% of ambulatory patients supported with current LVAD models when Rsp optimization is based on current clinical and echocardiographic recommendations.
- -
Age and postoperative BNP levels were independently associated with the degree of HLVU in ambulatory patients with cf-LVAD.
- -
BNP <300 pg/mL was able to predict freedom from high LV filling pressure with a negative predictive value of 86%.
- -
An optimal HLVU after LVAD implantation translated into more favorable effects on reverse myocardial remodeling.