To the Editor:
We report the case of a 72-year-old woman diagnosed with ostium secundum atrial septal defect (OS-ASD) in an echocardiogram performed as part of the work-up for her high blood pressure. Over time, progressive dilation of the right ventricle was observed with progressive functional deterioration, indicating closure of the defect. Transesophageal echocardiography (TEE) confirmed an OS-ASD of 15´12 mm and it was determined that the defect was amenable to closure using a percutaneous device.
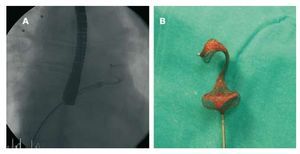
The procedure was performed under general anesthesia and TEE monitoring. Vascular access was via the right femoral vein. The size of the occluder device was defined using a balloon catheter with the stop-flow technique, whereby the diameter of the occluder device is chosen to correspond to balloon diameter at the moment an absence of shunt is observed. Based on this technique, it was decided to use a 24 mm Amplatzer type device (Amplatzer Septal Occluder, AGA Medical, Golden Valley, Minnesota, USA). The discrepancy with the initial size of the ASD was due to an excessively mobile posterior edge. In our laboratory we usually use a wider sheath diameter than that recommended by the manufacturer, but the appropriate size was not available. We therefore opted for a delivery system with a 9F sheath (diameter recommended by the manufacturer). However, after inserting the device a few centimeters, significant resistance was encountered and the device had to be retracted. It was also impossible to progress using a 10F sheath (Superarrow Flex, Arrow Inc., Reading, Pennsylvania, USA), so we eventually decided to switch to a 13F sheath (Mullins, Cook Europe, Bjaeverskov, Denmark). It was possible to advance with this sheath without encountering resistance; however, when the device was deployed distally in the left atrium, a 'cobra-like' configuration was observed (Figure 1A). This configuration was maintained after retracting the device (Figure 1B), although by slightly stretching the distal portion it was possible to regain the usual shape. The device was reintroduced without problems but again assumed a 'cobra-like' configuration. However, by making small back-and-forwards movements in the sheath it was possible to normalize the shape of the device and successfully close the atrial defect (Figure 2).
Figure 1. A: view by fluoroscopy of the 'cobra-like' configuration of the Amplatzer. B: image of the device after extraction for remodeling.
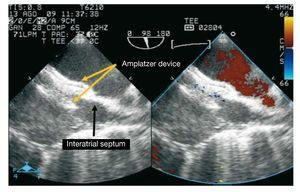
Figure 2. Transesophageal echocardiography image showing the Amplatzer device correctly positioned (yellow arrows) in the atrial septum (black arrow) after correcting the deformity and without residual short circuit.
The 'cobra-like' configuration of the Amplatzer device is a rare but known complication of percutaneous treatment of atrial septal defects that occurs in 0-3% of published series.1,2 It is in fact the extreme variant of a series of distortions deriving from a change in position of the device's nitinol wires. These distortions range from a slight bulge to the 'cobra-like' position. Several theories have been postulated about its cause, including the presence of a manufacturing defect, the excessively distal release of the device, which would result in adherence to the free wall of the left atrium, the left atrial appendage or the pulmonary vein orifice and the deformation of these,3 or difficulties in positioning the device within the sheath.4 We believe the present case is a clear illustration of the latter and hypothesize that the difficulty in advancing the device would have produced an anomalous crossover of the nitinol wires, with the resulting anomalous configuration during the device's self-expansion. The back-and-forward maneuvers within the sheath may have favored relocation of the wires, leading to recovery of the usual configuration. We recommend respecting the policy of slightly increasing sheath diameter to avoid forcing their advance. On the other hand, if the complication does occur, it is possible to remodel the device and place it successfully.