There is a paucity of information about the real benefit of colchicine administration in the first episode of acute idiopathic pericarditis (AIP). The main objective of the present study was to assess the real efficacy of colchicine in patients with AIP who did not receive corticosteroids.
MethodsRandomized multicenter open-label study. Patients with a first episode of AIP (not secondary to cardiac injury or connective tissue disease) were randomized into 2 groups: group A received conventional anti-inflammatory treatment plus colchicine for 3 months, and group B received conventional anti-inflammatory treatment only. None of the patients received corticosteroids. The primary endpoint was the appearance of recurrent episodes of pericarditis. The secondary endpoint was the time to first recurrence. Follow-up was extended to 24 months.
ResultsA total of 110 patients (83.6% men, age 44±18.3 years) were randomized to group A (n=59) and group B (n=51). No differences were found in baseline demographics or in the clinical features of the index episode or in the type of anti-inflammatory treatment administered in both groups. The follow-up was completed by 102 patients (92.7%). No differences were found in the rate of recurrent pericarditis between groups (12 patients [10.9%]; group A vs group B, 13.5% vs 7.8%; P=.34). The time to first recurrence (group A vs group B, 9.6±9.0 vs 8.3±10.5 months; P=.80) did not differ between groups.
ConclusionsAmong patients with a first episode of AIP who had not received corticosteroids, the addition of colchicine to conventional anti-inflammatory treatment does not seem to reduce the recurrence rate. Clinical trial registration: URL: https://www.clinicaltrialsregister.eu. Identifier: EudraCT 2009-011258-16
Keywords
Recurrent pericarditis is probably the most troublesome complication of acute idiopathic pericarditis (AIP) and represents one of the greatest therapeutic challenges among the disorders of the pericardium. The frequency of recurrences in clinical series including more than 40 patients1–6 varies between 8% and 80% (average 24%). Until now, no medical treatment has been shown to be unquestionably effective for the treatment of recurrences,6–8 although in recent years colchicine has appeared as a useful drug.9–15 As recurrences of pericarditis are probably related to an autoimmune response starting around the first episode of acute pericarditis, it seems logical to try to avoid this initial stimulus by using an anti-inflammatory/immunosuppressive drug such as colchicine. In a previous study, the COPE trial16 addressed the possible usefulness of colchicine when added to conventional treatment of the first episode of acute pericarditis. In that study, colchicine seems safe and efficacious in the treatment of the first episode as well as in the prevention of recurrences. However, the COPE trial was hampered by the fact that 15.8% of the patients were prescribed corticosteroid therapy, a factor that has been shown to increase the probability of recurrences6–8,17,18 and, therefore, could introduce some bias in the results of the study. In the more recent ICAP (Investigation on Colchicine for Acute Pericarditis) study,17 colchicine, when added to conventional anti-inflammatory therapy in patients with a first episode of acute pericarditis, also significantly reduced the rate of incessant or recurrent pericarditis. In this important multicenter, double-blind trial, the rate of prednisone use was between 5% and 8.3%, postcardiac injury pericarditis accounted for around 20% of patients, and a surprisingly high rate of incessant and recurrent pericarditis (37.5%) was observed in the placebo group.
Accordingly, we designed a similar study in a group of patients with a first episode of acute idiopathic pericarditis with the aim of investigating the real benefit of colchicine administration in patients who had not previously received corticosteroids, attempting to collect information on whether routine use of colchicine is warranted in this specific context.
METHODSTrial DesignA randomized multicentre open-label clinical trial with 2 parallel groups was conducted at 3 Spanish tertiary teaching centers. The study compared the efficacy of 2 treatment regimens in patients with a first episode of AIP to avoid recurrent pericarditis: conventional anti-inflammatory treatment with aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) plus colchicine (1 mg/12 h for patients who weighed more than 70 kg or 0.5 mg/12h in patients with a weight <70kg) for 3 months (group A) vs conventional antiinflamatory treatment (group B).
The inclusion criteria were: a) AIP evident at the time of inclusion, and b) agreement to participate in the study with provision of informed consent. The exclusion criteria were: a) pregnant or nursing women or women of childbearing potential not protected by a contraception method; b) life expectancy of less than 18 months; c) current treatment with corticosteroids or underlying diseases requiring treatment with corticosteroids; d) tuberculosis, malignancy, or bacterial pericarditis; e) postcardiac injury pericarditis; f) creatinine levels greater than 2.5mg/dl; g) liver disease or transaminase> 1.5 the upper limit of the normal; h) blood dyscrasia; i) contraindication or hypersensitivity to colchicine.
Study PopulationThe trial included 110 patients, of whom 50% were assigned to receive aspirin or NSAIDs and the other 50% to receive aspirin, NSAIDs, and colchicine for months.
This study complies with the Declaration of Helsinki. The study was approved by the Vall d’Hebron University Hospital Institutional Review Board and was registered in the EU Clinical Trials Register (EudraCT Number: 2009-011258-16). Prior to participating in this study, each investigator obtained local ethics committee approval and patients gave their written informed consent.
ProceduresAll patients received conventional treatment consisting of aspirin at initial doses of 1g every 6 or 8 hours, ibuprofen 600 mg every 8hours or indometacin 50mg every 8hours for 2 to 10 days with tapering over 3 to 4 weeks. In the group assigned to colchicine (group A), in addition to conventional treatment, colchicine was started at diagnosis of AIP. In all patients, treatment was started within the first week of symptom onset. Colchicine was administered at a dose of 1 mg/12h in patients> 70 kg or 0.5mg/12h in patients <70 kg for 3 months, reduced to half the dose if there was diarrhea. Corticosteroids were not administered in any of the patients. Other cardiac medications were prescribed at the discretion of the attending physician. Relapses were treated with aspirin or ibuprofen at the same doses as those used in the first episode. We strongly advised against the use of steroids. Colchicine was maintained for 3 months irrespective of the presence of recurrences. All patients received a proton pump inhibitor as gastroduodenal prophylaxis.
In addition to hematological and biochemical determinations, serial measurements of CK-Mb and troponin T were performed. Echocardiography was performed during patient admission. If pericardial effusion was documented, echocardiographic monitoring was performed according to clinical judgment.
Patients were deemed to have withdrawn from the study under 2 circumstances: a) no adherence with 24 months follow-up, b) following the patient's wishes or if violations of the monitoring protocol were evident. Patients who discontinued colchicine treatment due to adverse effects were followed up until the 24-month follow-up protocol was complete.
DefinitionsAcute pericarditis is diagnosed on the basis of the presence of 2 of the following criteria: typical pericarditic chest pain, pericardial rub and/or evolving repolarization abnormalities typical of acute pericarditis in an electrocardiogram (ECG). Idiopathic pericarditis is diagnosed when no underlying disease, previous cardiac injury or specific infectious etiology are identified after the applications of a protocol for management of pericardial diseases.19Recurrent pericarditis is diagnosed by a documented first episode of acute pericarditis, a symptom-free interval of 6 weeks or longer, and evidence of subsequent recurrence of pericarditis. Patients with persistent pericarditis or those with a symptom-free interval of less than 6 weeks are diagnosed with incessant pericarditis. Recurrence is diagnosed by recurrent typical pericarditic chest pain and 1 or more of the following signs: pericardial friction rub, evolving ECG changes, or echocardiographic evidence of a new pericardial effusion.
OutcomesThe primary end point of the study was to assess whether early administration of colchicine in the first episode of AIP may be effective in preventing recurrent episodes of pericarditis. The secondary study end point was to evaluate the time to the first recurrence and whether the potential beneficial effect of colchicine was limited to the period of its administration or was maintained after discontinuation of the treatment.
The safety of colchicine was assessed by the registering all the adverse events that appeared during its administration.
Sample SizeThe expected rate of recurrences in the group not exposed to colchicine was assumed to be 30%. Expected absolute reduction in the number of recurrences was 20%. Assuming a type I error of 5% and type II error of 20%, 49 patients per arm were required to obtain a power of 80%. Estimating a 10% loss to follow-up, the required sample size was calculated to be of 110 patients.
RandomizationPatients who met a*ll the inclusion criteria and none of the exclusion criteria were randomized in consecutive order of qualification to receive colchicine in addition to conventional antiimflammatory treatment or only conventional antiimflammatory treatment (1:1). Overall, randomization was conducted in permuted blocks with a block size of 4, through a web-based system (computer-generated sequence allocation), which was also used for electronic data capture throughout the study. Time zero was defined as the time of randomization. Patients were considered enrolled in the study and eligible for final intention-to-treat analysis.
Follow-upAll patients were followed up for at least 24 months after inclusion in the study. Follow-up visits to the outpatient clinic were scheduled at 6 weeks, 3, 6 and 12 months and thereafter until the end of the study. A minimum follow-up of 24 months for all patients was predetermined and the follow-up ended at December 2013. Each visit was performed through a structured interview (questionnaire) where the occurrence of symptoms or signs of a possible new episode of pericarditis were recorded. Assessment of treatment adherence was asked from each patient. Currently, there are no analytical methods that allow measuring levels of colchicine in serum or other body fluids. During follow-up, testing of each visit included basic vital signs and a general physical examination. All adverse events were monitored and recorded.
Statistical AnalysisAll analyses were carried out by intention-to-treat. Quantitative variables are expressed as mean±standard deviation and category variables are expressed as the absolute value of cases and the percentage (%). For mean comparisons, the Student t test was used, and the Mann-Whitney U statistic was used when necessary. Comparative proportions analysis was performed using the chi-square test, and the Fisher test was used when necessary. Statistical significance was considered for a value <.05. The distribution of events (recurrent pericarditis) was estimated by Kaplan-Meier analysis and compared groups by log-rank test. A logistic regression analysis was carried out to assess possible contribution of other risk factors for recurrences including sex, previous use of colchicine, and pericardial effusion at presentation. All analyses were performed using SPSS 21.0.
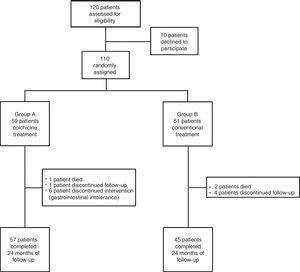
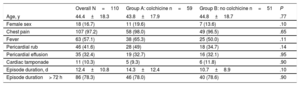
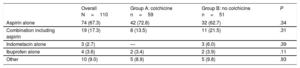
RESULTSBaseline CharacteristicsFrom February 2004 to May 2010, 110 patients (83.6% men, mean age 44.4±18.3 years; median age 40 [range, 24-67] years) with a first episode of AIP who were candidates to participate in the current study were randomized to each treatment group. Group A comprised 59 patients who received conventional treatment plus colchicine for 3 months and group B comprised 51 patients who received conventional treatment alone. Of the 110 randomized patients, 102 (92.7%) completed the follow-up and were analyzed. Three patients died before the first year of follow-up because of sudden cardiac death (1 patient from group A) and neoplasia of the colon and pancreas respectively (2 patients from group B), and 5 patients (1 from group A and 4 from group B) were lost during follow-up (Figure 1). No differences were found in baseline demographic characteristics or in the clinical features of the index pericarditis episode (Table 1). Conventional treatment was similar in both groups, with aspirin alone (67.3%) or in combination with other analgesic and/or anti-inflammatory drugs (17.3%) as the main treatment (Table 2). No patient received corticosteroid treatment.
Baseline Demographic and Clinical Characteristics
| Overall N=110 | Group A: colchicine n=59 | Group B: no colchicine n=51 | P | |
|---|---|---|---|---|
| Age, y | 44.4±18.3 | 43.8±17.9 | 44.8±18.7 | .77 |
| Female sex | 18 (16.7) | 11 (19.6) | 7 (13.6) | .10 |
| Chest pain | 107 (97.2) | 58 (98.0) | 49 (96.5) | .65 |
| Fever | 63 (57.1) | 38 (65.3) | 25 (50.0) | .11 |
| Pericardial rub | 46 (41.6) | 28 (49) | 18 (34.7) | .14 |
| Pericardial effusion | 35 (32.4) | 19 (32.7) | 16 (32.1) | .95 |
| Cardiac tamponade | 11 (10.3) | 5 (9.3) | 6 (11.8) | .90 |
| Episode duration, d | 12.4±10.8 | 14.3±12.4 | 10.7±8.9 | .10 |
| Episode duration> 72 h | 86 (78.3) | 46 (78.0) | 40 (78.6) | .90 |
Values are expressed as No. (%) or mean±standard deviation.
Treatment
| Overall N=110 | Group A: colchicine n=59 | Group B: no colchicine n=51 | P | |
|---|---|---|---|---|
| Aspirin alone | 74 (67.3) | 42 (72.8) | 32 (62.7) | .34 |
| Combination including aspirin | 19 (17.3) | 8 (13.5) | 11 (21.5) | .31 |
| Indometacin alone | 3 (2.7) | — | 3 (6.0) | .39 |
| Ibuprofen alone | 4 (3.6) | 2 (3.4) | 2 (3.9) | .11 |
| Other | 10 (9.0) | 5 (8.9) | 5 (9.8) | .93 |
Data are expressed as No. (%).
Overall, the average follow-up was 30.2±17.0 [range, 24-145] months. No differences were found in the mean follow-up (group A vs group B, 28.4±15.3 vs 30.5±18.6 months; P=.5) or in the percentage of patients who ended the 2 year follow-up in each group (group A vs group B, 93.2% vs 92.1%; P=.88).
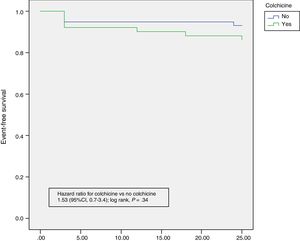
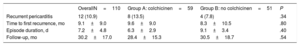
OutcomesRecurrent pericarditis occurred in 12 patients (10.9%). No episodes of incessant pericarditis occurred in any of the patients. The main results for each group are summarised in Table 3. No statistically significant differences in recurrence rate were found between groups (group A vs group B, 13.5% vs 7.8%; P=0.34). Among the 12 patients who had recurrence of pericarditis, 7 occurred during the first 3 months (4 in group A and 3 in group B) (Table 1), 2 at 6 months, 1 at 12 months, 1 at 18 months, and 1 at 24 months of follow-up. The time to the first recurrence did not differ between groups (group A vs group B, 9.6±9.0 vs 8.3±10.5 months; P=.80). Colchicine administration did not result in a lesser severity of the index episode of pericarditis, as measured by pericardial effusion, cardiac tamponade, or episode duration (Table 1). No patient had more than 1 recurrence. Symptom severity (symptom duration, presence of pericardial effusion or tamponade) of the recurrent episodes of pericarditis were no different between the groups (Table 2). No patient experienced a severe pericardial effusion or cardiac tamponade during episodes of recurrence. Kaplan-Meier survival curves showed no significant differences for subsequent recurrences (Figure 2). Multivariate analysis showed no predictors for episodes of recurrences.
Main Outcomes
| OverallN=110 | Group A: colchicinen=59 | Group B: no colchicinen=51 | P | |
|---|---|---|---|---|
| Recurrent pericarditis | 12 (10.9) | 8 (13.5) | 4 (7.8) | .34 |
| Time to first recurrence, mo | 9.1±9.0 | 9.6±9.0 | 8.3±10.5 | .80 |
| Episode duration, d | 7.2±4.8 | 6.3±2.9 | 9.1±3.4 | .40 |
| Follow-up, mo | 30.2±17.0 | 28.4±15.3 | 30.5±18.7 | .54 |
Data are expressed as No (%) or mean±standard deviation.
Adverse effects related directly to colchicine were observed in 8 patients (13.5% of the colchicine group); all of them had diarrhea. In 2 patients, diarrhea was resolved by reducing the colchicine dose but it had to be discontinued in 6 patients. In 1 additional case, the patient decided to discontinue colchicine of his own initiative, although no adverse effect was observed. In the conventional treatment group, gastrointestinal disorders occurred in 4 patients (8.5%) with no patient with diarrhea and no need for treatment discontinuation because of adverse effects. No serious adverse effects where observed in either group.
DISCUSSIONThe main finding of our study is that the incidence of recurrence was very low after a first episode of AIP in patients who had not received corticosteroids, and was no different in patients who received colchicine in addition to conventional anti-inflammatory treatment compared with patients who only received conventional anti-inflammatory treatment. The clinical severity of the first episode of pericarditis (symptom duration, presence of moderate or severe pericardial effusion or tamponade) and subsequent recurrences did not differ between the 2 groups. In addition, no predictors of recurrences were identified on multivariate analysis.
Patients included in the present study were patients who received a diagnosis of AIP following strict criteria. Importantly, all patients had typical pericardial chest pain and nearly half had pericardial rub. The prevalence of pericardial effusion was lower than that observed in other studies, such as the ICAP trial17 (which also include patients with connective tissue disease and postcardiac injury syndrome). This may be related to the inclusion and diagnostic criteria used in the different series, and also to the fact that a minimum pericardial effusion may not have been registered in some patients in our study. Irrespective of these possibilities, the presence of pericardial effusion was not identified as a predictor for recurrences in the series published in the literature.7,16–18 Thus, we believe that our cohort is representative of the patients who consulted for AIP and did not select a group at lower risk of recurrences.
The time to first recurrence did not differ between the 2 groups (group A vs group B, 9.6±9.0 vs 8.3±10.5 months; P=.80). Interestingly, most of the recurrences occurred in the first 3 months (4 in group A, 3 in group B), suggesting that in these patients, colchicine was not efficacious even during its administration (in group A).
Our study had an unexpected lower incidence of recurrences (10.9%) compared with previous studies. Patients on colchicine had 13.5% of recurrences compared with 7.8% in patients who received conventional anti-inflammatory treatment alone. In contrast, in the COPE trial,16 the recurrence rate at 18 months was much higher in patients without colchicine treatment (10.7% in the group on colchicine vs 32.3% in the group that received conventional anti-inflammatory drugs alone). Furthermore, the more recent multicenter, double-blind ICAP (Investigation on Colchicine for Acute Pericarditis) study17 showed similar findings. Colchicine, when added to conventional anti-inflammatory therapy, significantly reduced the rate of incessant or recurrent pericarditis from 37.5% in the placebo group to 16.7% in the colchicine group (relative risk reduction in the colchicine group of 0.56). In addition, in the COPE trial, colchicine reduced the persistence of symptoms at 72 hours and prolonged the symptom-free interval between recurrences. An important point in this study is that corticosteroid therapy was prescribed in 19 patients (15.8%), a factor that can increase the rate of recurrences. In fact, the recurrence rate at 18 months was as high as 86.7% in the group of patients who received prednisone, and this was the subgroup that seemingly obtained the most benefit from the addition of colchicine (recurrence rate of 11.1%). Of note, in the ICAP trial, the rate of prednisone use was between 5% and 8.3%, and a surprisingly high rate of incessant and recurrent pericarditis (37.5%) was observed in the placebo group. In addition, connective tissue diseases and postcardiac injury pericarditis accounted for 15.8% and 23.5% of cases in the COPE and ICAP study, respectively.
The design of our study is very similar to that of the COPE and ICAP studies. The most relevant difference is that in our study, we only included patients with acute idiopathic/viral pericarditis who had not received corticosteroids due to their possible harmful influence, previously mentioned. Patients with postcardiac injury pericarditis or pericarditis secondary to connective tissue disease were not included in our study. In addition, in our study protocol, higher doses of colchicine were included compared with those adopted in previous trials and suggested by the 2015 European Society of Cardiology guidelines,20 in order to guarantee the clinical effect of colchicine.
However, some differences in the results of the 2 studies are difficult to explain. For instance, in the COPE trial, the recurrence rate in the subgroup of patients who received conventional anti-inflammatory treatment alone was 23.5%, higher than the figure in our study (7.8%). This important difference cannot be explained by the treatment regimen as in both studies aspirin was administrated in similar doses and was tapered over 3 to 4 weeks. Possibly, the inclusion of patients with postpericardiotomy syndromes and connective tissue diseases in the COPE trial and in the ICAP study could have had some influence on the higher recurrence rate, as the recurrence rate can be higher in these specific etiologies of pericarditis.16,17 Another possible explanation relates to the criteria used to establish the diagnosis of incessant or recurrent pericarditis. In the COPE trial, acute pericarditis (first episode) was diagnosed when at least 2 of the 3 established criteria (typical chest pain, pericardial friction rub, and widespread ST-segment elevation on the ECG) were present. However, in this study no mention is made of whether the same criteria were required for diagnosis of the recurrences. It is well known that after an episode of acute pericarditis, some patients present with persistent or relapsing chest pain but without other criteria to establish a diagnosis of acute pericarditis.18 In our study, we were strict in requiring the same criteria for the first episode and for recurrences.
Recurrent pericarditis is one of the most tedious complications of acute pericarditis and it represents one of the greatest challenges in the treatment of pericardial disorders. Due to the apparent benefit of colchicine in the treatment of relapsing pericarditis, it seems tempting to administer this drug in the first episode of acute pericarditis. The rationale would be to try to avoid the immune response that triggers the mechanism responsible for subsequent recurrences. Colchicine acts through inhibition of various leukocyte functions through its capacity to bind beta-tubulin and to disrupt microtubules. On the basis of the COPE and ICAP studies and meta-analysis,17,18,21,22 colchicine has been recommended as class I with level of evidence A in the first episode of acute nonbacterial pericarditis in the last European Society of Cardiology guidelines for the diagnosis and management of pericardial diseases.20 However, the findings of our study cast doubt on the validity of this categorical recommendation in patients with AIP.
LimitationsThis study has several limitations. First, we calculated a sample of 55 patients in each group assuming a recurrence rate of 30%, in order to show a reduction of 20% in the incidence of recurrences with the administration of colchicine. With that figure, the statistical power was 80%. The unexpected lower incidence of recurrences accounts for the loss of any statistical power to demonstrate a possible beneficial effect of colchicine in the prevention of recurrences. Second, in our study, the frequency of pericardial effusion was rather low (around 30%). This may be because, in our study, pericardial effusion was not considered an inclusion criterion for the diagnosis of acute pericarditis, and therefore a minimal pericardial effusion may not have been registered in some cases. Third, in all patients, treatment was started within the first week of symptoms. Fourth, we cannot provide information about whether patients had elevated C-reactive protein and inflammatory markers. Finally, this study was an open-label study, and we accept the inherent limitations of nonblinded studies.
CONCLUSIONSThe present study shows that the recurrence rate after a first episode of acute idiopathic pericarditis is lower than previously reported when no corticosteroids are administered. In this specific context, colchicine administration does not seems to have any relevant influence on the recurrence rate or on the severity of clinical manifestations. Accordingly, we believe that systematic administration of colchicine is not warranted in a first episode of acute idiopathic pericarditis. A further study with an extended sample size is warranted to validate our data.
FUNDINGThis study was supported by Instituto Carlos III of the Government of Spain (PI 09/1478), CIBERCV and by the European Regional Development Fund.
CONFLICTS OF INTERESTNone declared.
- –
Recurrent pericarditis is probably the most troublesome complication of acute pericarditis and represents one of the greatest therapeutic challenges among the disorders of the pericardium.
- –
Colchicine may be useful to treat recurrent pericarditis and to prevent new recurrences in some cases.
- –
In our environment, the incidence of recurrences was very low after a first episode of acute idiopathic pericarditis in patients who had not received corticosteroids (10.9%).
- –
In this specific context, colchicine administration did seem to have any relevant influence on the recurrence rate or on the severity of clinical manifestations.