The new edition of the clinical practice guidelines of the European Society of Cardiology on valvular heart disease1 is an update of the previous 2017 guidelines. This revision is justified by major epidemiological changes (a growing incidence of degenerative etiologies vs rheumatic), increasingly robust clinical evidence on the value of molecular and imaging (3D echocardiography and cardiac magnetic resonance imaging) biomarkers in decision-making, and, above all, outcomes from randomized clinical trials on the management of secondary mitral regurgitation (MR) with antithrombotic therapies in patients with surgical or transcatheter prosthetic valves and on risk stratification and optimal intervention timing.2 The resulting document is lengthy (54 pages of text followed by more than 550 references) and contains a table summarizing the new updated recommendations in this edition of the document (Table 3 of the guidelines). Here, we gather the aspects that we consider the most novel.
STRENGTHENING OF SPECIALIZED CENTERS AND SHARED DECISION-MAKINGThe document stresses the role of heart valve clinics in guaranteeing high-quality patient-centered care. In this regard, the authors list in detail the prerequisites of these centers. They should not only concentrate a large volume of activity in the different diagnostic and therapeutic procedures, but also undertake periodic audits of their outcomes. In addition, the need is reinforced for shared therapeutic decision-making among the various specialties and subspecialties involved in the process (Heart Team). For the first time, these professionals include nursing staff specialized in treating patients with valvular heart disease.
CHANGES IN EVALUATION CRITERIA AND THEIR INTERVENTION INDICATIONSThe guidelines highlight the role of echocardiography in the diagnosis and management of valvular heart diseases and the considerable additional value of 3D transesophageal echocardiography and computed tomography (CT) in the selection of patients for surgical or transcatheter treatment. 3D transesophageal echocardiography is very useful for detailing the valve anatomy and assessing repairability and is essential for guiding transcatheter mitral and tricuspid valve procedures. CT angiography is indispensable in the planning of transcatheter procedures to assess the vascular accesses and anatomical structures involved in each procedure.
The guidelines do not substantially modify the quantification indices of valvular heart diseases. The document underlines the possible limitations in the calculation of valve area in aortic stenosis3 and the importance of controlling blood pressure during the study. Also stressed is the value of CT in quantifying aortic valve calcification, particularly in patients with aortic stenosis with paradoxical low-flow, low-gradient aortic stenosis. In patients with aortic valve disease, the size of the ascending aorta must be assessed and CT is recommended when the ultrasound-determined diameter exceeds 40mm. The aorta measurement methodology is established and the maximum root diameter should be determined from the sinus-to-sinus diameter. In addition, the document stresses the importance of integrating various echocardiographic parameters and even considering the prognostic value of the effective regurgitant orifice in MR.
Although ventricular diameters and ejection fraction (EF) are good parameters for assessing left ventricular (LV) function, the guidelines recognize the potential usefulness of global longitudinal strain, particularly when the EF values are borderline indicative of surgery. In this regard, cardiac magnetic resonance imaging can be hugely helpful when the ultrasound data are not definitive, particularly for assessing the right ventricle and measuring myocardial fibrosis.
INTERVENTION INDICATORS IN ASYMPTOMATIC PATIENTSIntervention decisions in asymptomatic patients with severe valvular heart disease are a major challenge. Generally, the decision is based on parameters indicating myocardial damage and associated with worse prognosis. In other words, we are already late, because these patients already have signs predicting a worse progress. Recent studies provide sufficient evidence to support stricter cutoff points, and this is reflected in the guidelines. An important aspect is the higher mortality in women with aortic stenosis vs men, which is probably related to delays in the diagnosis and intervention indication. A higher incidence has also been confirmed of heart failure after mitral valve repair in women, probably due to more advanced disease grades at surgery. These factors have particularly been considered in valve regurgitations.
Thus, in patients with severe asymptomatic aortic regurgitation, the recommendation based on end-diastolic LV diameter has been eliminated and the recommendation based on end-diastolic diameter (> 50mm/m2 or > 25mm/m2) has been upgraded from IIa to I, and surgery is recommended (IIb) if this diameter is > 20mm/m2. In addition, the guidelines maintain the recommendation based on LVEF (> 50%; class I, level of evidence B) but that of a LVEF <55% is included as a IIb recommendation. Similarly, in asymptomatic primary MR, a LVEF cutoff point of 60% remains a class I recommendation with level of evidence B, but the cutoff point of the LV end-diastolic diameter decreases from 45 to 40mm.
There are noteworthy changes in asymptomatic severe aortic stenosis. First, “surgical valve replacement” has been replaced with the term “intervention”, accepting the possibility that studies already underway might establish an indication for transcatheter interventions in asymptomatic patients. LVEF-based indications are stressed: the level of evidence for LVEF ≤ 50% increases (from C to B) and a IIa recommendation is included for LVEF ≤ 55%. The level of evidence increases from C to B for the remaining indicators of worse prognosis (very severe stenosis, severe calcification, progression, or B-type natriuretic peptide elevation). The definition of “very severe” is less strict (changing from a maximum velocity of 5.0 m/s to > 5.5 m/s). Although some studies show higher mortality in patients who undergo surgery with this degree of stenosis, the evidence of the relationship of the other parameters with prognosis is weak, given that these studies consider events to not only be mortality (rare), but also surgical intervention, which is the predominant event.4 A IIa recommendation with level of evidence B would thus appear to be too weak.
The guidelines maintain the indication for surgery in asymptomatic patients with severe valve disease if revascularization surgery of the ascending aorta or another valve is indicated. For patients with a noncardiac surgical indication, the specific guidelines should be consulted. In general, in asymptomatic patients, severe aortic stenosis is the valvular heart disease that causes more perioperative complications than noncardiac surgery. Thus, if the procedure is not urgent and the risk is high, aortic valve intervention should take precedence over noncardiac surgery.
INTERVENTION OPTIONS IN AORTIC STENOSISIn patients with symptomatic severe aortic stenosis, the guidelines maintain the same treatment recommendations for the various subgroups based on the mean transvalvular pressure gradient and cardiac output (aortic transvalvular flow).1 However, this new edition introduces changes in the mode of treatment. Randomized clinical trials in patients with symptomatic severe aortic stenosis and low or intermediate surgical risk have shown noninferiority regarding the safety and efficacy of transcatheter aortic valve implantation vs surgical replacement.5 In addition, transcatheter implantation tends to be superior to surgical implantation when the former is performed via transfemoral access. It is important to note that the patients with low and intermediate surgical risk included in these studies were predominantly men and that 40% were aged > 75 years. Excluded were patients with low cardiac output and unfavorable anatomical characteristics (bicuspid aortic valve or 3-vessel coronary heart disease). Consequently, the new document stresses the importance of a multidisciplinary (Heart Team) discussion of the clinical, anatomical, and procedural characteristics and, for the first time, explicitly recommends that the decision be made with the patient's participation. In younger patients (< 75 years) with low surgical risk (STS-PROM or EuroSCORE II <4%) and with no contraindications to surgery, surgical replacement of the aortic valve is assigned a class I recommendation. Surgery is also a class I indication in patients who have no contraindications to surgery but have contraindications to transfemoral implantation. In patients older than 75 years with high surgical risk (STS-PROM or EuroSCORE II <8%) or contraindications to surgery, transcatheter implantation is assigned a class I recommendation.6 For the remaining patients, both transcatheter and surgical implantation are awarded a class I indication. Transcatheter implantation by access other than transfemoral can be considered in inoperable patients when the transfemoral approach is not feasible (class IIb recommendation).7 The guidelines also stress that the relationship between the durability of the aortic bioprosthesis and patients’ life expectancy should always be considered (which is related not only to age, but also to sex, frailty, comorbidities, and risk of futility).
INTERVENTION OPTIONS FOR THE MITRAL VALVEThe new guidelines include major contributions regarding what are referred to as mitral valve intervention indications, specifically in MR. The expansion and consolidation of surgical repair techniques in degenerative disease and the excellent results in experienced centers have enabled the intervention indication to be performed sooner in asymptomatic patients with degenerative MR (see the section on the reduction in criteria for diastolic diameter). Notably, early interventions in asymptomatic patients are only justified when surgical repair has a high probability of being durable and can be conducted in an experienced center. The document underlines the importance of concentrating experience with valvular heart diseases and with distinct techniques to treat these patients.
In addition, progress in transcatheter MR repair techniques, mainly in transcatheter edge-to-edge repair (TEER), has been a real surprise in this edition of the guidelines. For primary MR, mitral valve surgery is the recommended treatment strategy, and TEER can now be considered (IIb recommendation) in patients considered inoperable after multidisciplinary evaluation. For symptomatic secondary MR despite optimal medical therapy, a new recommendation (class I) has been introduced for a valve intervention, either surgical or percutaneous, with a level IIa recommendation for the application of the TEER technique in patients with chronic secondary MR who are not candidates for surgery. The indication is limited to symptomatic patients with severe secondary MR (now defined in these guidelines by a 30mm2 effective regurgitant orifice and not by 20mm2, as in the previous document) who are already receiving optimal medical therapy that includes cardiac resynchronization if indicated. In patients with these characteristics and with concomitant coronary heart disease who require treatment and have high surgical risk, there is a new class IIa recommendation for transcatheter coronary treatment followed by TEER. This approach is also indicated in patients requiring transcatheter aortic valve implantation (TAVI). For patients who are to undergo coronary artery revascularization surgery, the guidelines continue to recommend (class I) concomitant mitral valve surgery in the case of severe secondary MR. The surgical technique should be individualized and consider the anatomy of the mitral valve and ventricles (repair, replacement). For patients with severe secondary MR with no other concomitant cardiac or coronary heart disease who remain symptomatic with optimal medical therapy, TEER is now the most robust indication, at class IIa (IIb before) for those contraindicated for surgery and with criteria predicting a good response to this treatment, such as very severe MR, less dilated ventricles, and LVEF > 30%.8 This indication is maintained at class IIb for other patients, except those with LVEF <15%. Thus, in this edition of the guidelines, surgical treatment is noted as a recommended option for secondary MR in patients with LVEF between 15% and 30%; this cutoff was set at 30% in the previous guidelines. Given that this is not based on new original evidence, this change in recommendation in surgery for secondary MR seems to involve extrapolation of the outcomes of the clinical trials with TEER to the surgical option.
MANAGEMENT OF THE TRICUSPID VALVEThe interest in the tricuspid valve arises in response to studies showing that functional or secondary tricuspid regurgitation (TR) continues to be an undertreated entity with poor prognosis. The guidelines stress the use of echocardiography to adequately quantify TR severity and insist on a multiparametric approach that involves multimodality imaging if necessary. The document stresses the superiority of a biplane evaluation or the 3D echocardiography-guided area of the vena contracta over the conventional 2D measurement and incorporates the grading terms “massive” and “torrential” due to their prognostic value. The surgical indications are maintained for symptomatic patients with stenosis or severe primary TR and patients undergoing left valve surgery. This indication exists because severe untreated TR might not improve after treatment of the left-sided valve with the reduction in the right ventricular afterload. Moreover, reintervention for recurrent TR is associated with higher mortality, and treatment of the tricuspid valve together with left-sided surgery is not linked to higher surgical risk.9 In addition, changes have been introduced in 2 other aspects: a) a tendency for an earlier operation in asymptomatic patients with severe primary TR who are considered suitable for surgery; and b) indications for transcatheter devices in patients with severe inoperable secondary TR.
Some important aspects of TR remain unresolved in this edition of the guidelines. The authors do not propose multiparametric approaches to TR quantification that integrate other clinical and echocardiographic parameters associated with prognosis (coarctation, annulus, pulmonary ventriculoatrial coupling).9 Cardiac magnetic resonance imaging can be more useful than indicated in the guidelines, particularly for evaluating right ventricular volumes and EF. The identification of cutoff points that better predict prognosis would probably improve the selection of the optimal time for surgery. There is no discussion of the role of invasive diagnosis in TR secondary to atrial fibrillation (AF) for revealing diastolic dysfunction or significant MR that is missed by the reduction in the LV preload secondary to TR. Diuretics can drastically decrease the severity of TR secondary to heart failure with reduced or preserved EF. In contrast to the ESC/EACTS document, the North American guidelines recommend gradual targeted medical therapy (IIa) and indicate the need to reevaluate the severity of the regurgitation after the optimization of medical therapy.10 Regarding the transcatheter repair of secondary TR, the characteristics of the potential candidates for these techniques are not clear.
ROLE OF DIRECT ORAL ANTICOAGULANTSA major contributor to the innovation in the present revision of the guidelines is concentrated in the antithrombotic therapy of patients with prosthetic valves. Regarding the prescription of antithrombotics to patients with valvular heart disease, the guidelines recommend direct oral anticoagulants (DOACs) in patients with bioprostheses and AF from 3 months after valve implantation. The safety and efficacy of DOACs in terms of bioprostheses in the first 3 months after surgical or transcatheter valve implantation are not clearly established. According to the guidelines, DOACs can be considered in the first 3 months after surgical implantation of a mitral bioprosthesis in patients with and without AF but the recommendation is class IIb and the level of evidence is C due to the small number of randomized patients in the RIVER11 and ENAVLE12 trials in the first 3 months after valve implantation. The RIVER trial (rivaroxaban vs warfarin) exclusively evaluated patients with a mitral prosthesis, and only the ENAVLE trial (edoxaban vs warfarin), with just 220 patients, included individuals with aortic bioprostheses. For this reason, in patients without a baseline indication for anticoagulation and with an aortic bioprosthesis, the guidelines recommend low-dose aspirin (75-100mg/d) or anticoagulation with vitamin K antagonists (VKAs) in the first 3 months after surgery (IIa recommendation). For patients with AF and native valve disease, DOACs are recommended in those with aortic stenosis, aortic regurgitation, or MR. Patients with mitral stenosis and AF should be anticoagulated with VKAs.
ANTICOAGULATION IN PATIENTS WITH TRANSCATHETER AND MECHANICAL PROSTHESESAnother of the major changes lies in the antithrombotic management of patients with transcatheter prostheses. Thus, according to the results of the POPular TAVI trial,13 long-term antiplatelet monotherapy is recommended (Ia) instead of the dual antiplatelet therapy indicated in the previous guidelines. If the patient has an indication for chronic anticoagulation, the type of anticoagulant to use is unclear, given the absence of evidence. After the publication of these guidelines, the outcomes have been published of the ENVISAGE study, which found noninferiority of edoxaban vs VKAs in this group of patients but higher bleeding risk in the DOAC group.14
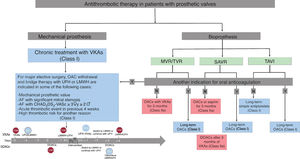
For patients with mechanical prostheses, the indication is still treatment with VKAs. The new recommendations are concentrated on treatment during the perioperative period. Although based on expert consensus (level C), major points are clarified, such as when to discontinue VKA therapy, to which patients to prescribe bridge therapy with heparin, when to restart anticoagulation in the postoperative period, and the selection of the concomitant antiplatelet therapy (figure 1). In this regard, bridge therapy is surprisingly recommended at lower CHA2DS2-VASc scores than in other consensus documents of the European Society of Cardiology.
Anticoagulation in patients with surgical and transcatheter prosthetic valves. DOACs, direct oral anticoagulants; LMWH, low-molecular-weight heparin; MVR, mitral valve replacement or repair; OACs, oral anticoagulants; SAVR, surgical aortic valve replacement; TAVI, transcatheter aortic valve implantation; TVR, tricuspid valve replacement or repair; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Finally, in line with the recommendations of the recent guidelines for the treatment of acute coronary syndrome,15 in patients undergoing PCI with indication for anticoagulation, the triple antithrombotic therapy time has been reduced to 1 week (Ib), with the subsequent treatment with clopidogrel maintained for 6 to 12 months, based on thrombotic risk. Nevertheless, these recommendations must always be individualized.
SELECTION OF PROSTHETIC VALVES AND ATRIAL APPENDAGE CLOSURE PROCEDURESThe guidelines recommend valve repair over replacement with prosthesis, as long as the outcomes can be predicted to be long-lasting and functionally effective (particularly in MR). Regarding the selection of the type of prosthesis that should be implanted when required, the guidelines continue to strongly recommend (class I) that the decision be made in accordance with patients’ preferences and lifestyle and by considering aspects beyond age and life expectancy and prosthesis durability. The presence of any formal contraindication to oral anticoagulation with VKAs rules out the use of mechanical prostheses, given that DOACs are officially contraindicated with this type of prosthesis. Patients’ chronological age or life expectancy have a lower level of recommendation (class IIa) and must be balanced against the estimated durability of the biological prosthesis (at least 10 years for surgical and not established for transcatheter). The recommendation is maintained for mechanical prostheses in individuals younger than 60 years in the aortic position and younger than 65 years in the mitral position, as well as bioprostheses in individuals older than 65 years in the aortic position and older than 70 years in the mitral position.
However, given the lack of randomized and comparative evidence, the guidelines do not include other potentially relevant and undoubtedly controversial issues. The possibility of transcatheter implantation in degenerated bioprostheses (as long as the size is sufficient) would decrease intervention risk and would possibly allow a lowering of the age of recommendation of the first bioprosthesis implant. Nonetheless, surgical explantation of a transcatheter prosthesis is an extremely complicated procedure, which is why bioprostheses are not recommended as the initial replacement prosthesis in very young patients. The usefulness of low-dose DOACs after mechanical aortic prosthesis implantation remains unknown, with ongoing clinical trials (NCT04142658) that, in the case of positive outcomes, will also permit modification of the age of indication of this type of prosthesis.
Regarding left atrial appendage closure during the surgical procedure, the recommendation has been increased (from class IIb to IIa) in patients with CHA2DS2-VASc > 2 and AF, based largely on the results of the LAAOS III clinical trial.16 After any type of cardiac surgery, left atrial appendage closure reduces the occurrence of stroke and systemic embolisms. The atrial appendage closure methods were clearly specified in the cited study, and the accepted approaches were amputation and closure, direct internal suture, or the use of specific occlusion devices. Explicitly not accepted was atrial appendage closure using simple ligation; thus, this method should be avoided.
MANAGEMENT OF PROSTHETIC DYSFUNCTIONThe guidelines maintain the recommendation for annual clinical follow-up and echocardiography in stable patients with a bioprosthetic valve (surgical or transcatheter). The document stresses the use of different imaging techniques for suspected prosthetic dysfunction, beginning with transthoracic echocardiography. Systematic transesophageal echocardiography is recommended for suspected thrombosis, endocarditis, or paravalvular leak (particularly in the mitral position). Fluoroscopy or, failing that, CT, if available, can evaluate the kinetics of leaflet occluders. CT plays a major role in the differential diagnosis of thrombus and pannus.
Surgery is the first option for the treatment of bioprosthetic dysfunction. However, transcatheter valve-in-valve treatment can be an alternative in high-risk patients, whether in the aortic position via a transfemoral approach with favorable anatomical characteristics (IIa B) or in the mitral and tricuspid positions (IIb B). Detailed planning is required to avoid complications such as coronary obstruction or LV outflow tract obstruction. Valve-in-ring treatment can be acceptable in selected patients, particularly in the mitral position, and should be individualized in each case by the multidisciplinary team.
The treatment of choice for periprosthetic dehiscence continues to be surgery in the presence of endocarditis or hemolysis with severe symptoms, but the level of recommendation for transcatheter closure has increased to IIa B in symptomatic patients with high surgical risk.
The acute management of prosthetic thrombosis is unchanged from the previous guidelines. In general terms, surgery is the preferred option for obstructive thrombosis in critically ill patients or for nonobstructive thrombosis with thrombus > 10mm or embolic phenomena. Fibrinolysis is reserved for patients when surgery is not possible, for patients with very high surgical risk, and for thrombosis in right-sided prostheses. Indefinite anticoagulation is recommended after a biological valve thrombosis event. Although CT after TAVI reveals an elevated prevalence of hypoattenuated leaflet thickening, its significance is uncertain. If this finding is accompanied by reduced leaflet motion and elevated gradients, full-dose anticoagulation can be considered.
MANAGEMENT OF VALVULAR HEART DISEASES IN PREGNANCYThere are no major changes from the previous guidelines concerning the management of valvular heart diseases during pregnancy. The guidelines once again highlight the value of discussing the approach to valvular heart diseases before and during pregnancy in a team including anesthesiologists, obstetricians, and neonatologists. The authors remind us that, in women with a mechanical valve, particularly in the mitral position, pregnancy is associated with a high risk of maternal and fetal complications requiring careful management, with the patient taking precedence over the pregnancy. Specific recommendations are made regarding the management of mitral stenosis with an area <1.5cm2 during pregnancy. In symptomatic severe aortic stenosis, aortic valvuloplasty is recommended when symptoms persist despite medical therapy. The guidelines reiterate the promising role of TAVI in this context. Due to the high fetal mortality, surgery under cardiopulmonary bypass should be restricted to cases in which transcatheter catheter is not possible or has failed or in which the mother's life is at risk. Specific recommendations are made regarding cesarean delivery in patients with severe aortic or mitral stenosis, an ascending aorta diameter > 45mm, or severe pulmonary hypertension or in patients whose delivery starts while they are being treated with VKAs or less than 2 weeks after their discontinuation. Recommendations remain concerning anticoagulation in pregnant women with mechanical prostheses and the authors note that these patients should be followed up in specialized centers.
AREAS WITH GAPS IN EVIDENCE AND OMITTED ISSUESAs in previous editions, the guidelines gather in a specific section the areas in which the scientific evidence is insufficient to make solid recommendations. In terms of patients with aortic stenosis, the authors highlight the lack of information on the safety and effectiveness of DOACs in patients with valve replacement in the 3 months after the procedure, the absence of studies comparing the durability of surgical and transcatheter prostheses, the role of interventions in asymptomatic patients, and that of revascularization in patients with severe stenosis and underlying asymptomatic coronary heart disease. In the field of MR, the information deficit is centered on the long-term outcomes of transcatheter interventions, the indications for these techniques in patients with severe primary regurgitation and low surgical risk, and the potential survival benefit of valve interventions (transcatheter or surgical) in patients with severe secondary regurgitation. Regarding TR, controversy still surrounds the time for surgery in patients with primary disease and the approach for secondary disease. In addition, very few clinical trials have assessed the safety and efficacy of emerging transcatheter treatment techniques.
The guidelines stress the systematic use of conventional cardiac stress tests in the follow-up of asymptomatic patients with valvular heart disease. However, no reference is made to the potential advantages of cardiopulmonary exercise testing in this group of diseases. Also omitted is the indication for cardiac rehabilitation after valvular heart disease correction, despite the favorable evidence.17
CONCLUSIONSThis update of the guidelines arrives at a transitional moment when the outcomes are being incorporated of multiple randomized clinical trials in topics such as antithrombotic therapy after intervention or transcatheter mitral repair. However, the lack of very long-term evidence (> 5 years) still impedes an unequivocal determination of the role of TAVI techniques in young low-risk patients. Despite the advances in imaging techniques in recent years, the guidelines highlight the central role that is still played by history-taking, physical examination, conventional echocardiography, and cardiac stress tests (in asymptomatic patients) in the diagnosis and follow-up of patients with valvular heart diseases. Patient empowerment is necessary when the correction indications are being considered. Thus, this new edition of the document stresses the essential role of professionals in helping patients to participate in the therapeutic decisions affecting them. The combination of their preferences with objective aspects such as age and sex, comorbidities, frailty, and the potential futility of the treatment must be integrated under shared decision-making conditions.
FUNDINGNone.
CONFLICTS OF INTERESTThe conflict of interest declaration documents of all authors can be seen in the supplementary data.
SEC Working Group for the 2021 ESC/EACTS guidelines for the management of valvular heart disease: Pablo Avanzas (coordinator), Javier Bermejo (coordinator), Manuel Barreiro-Pérez, Belén Cid, Victoria Delgado, J. Alberto San Román, Arturo Evangelista, Pastora Gallego, Francisco Javier García Aranda, José López-Menéndez, Marta Sitges, and Isidre Vilacosta.
SEC Guidelines Committee: Pablo Avanzas, Gemma Berga Congost, Araceli Boraita, Héctor Bueno, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos, Ignacio Ferreira-Gonzalez, Juan José Gomez Doblas, Domingo Pascual Figal, Antonia Sambola, Ana Viana Tejedor, José Luis Ferreiro (copresident), and Fernando Alfonso (copresident).
Supplementary data associated with this article can be found in the online version, at http://dx.10.1016/j.rec.2021.11.024
The names of all the authors of the article are listed in alphabetical order in Appendix 1.